Solids in which the atoms, ions, or molecules are arranged in a definite repeating pattern are known as crystalline solids. Metals and ionic compounds typically form ordered, crystalline solids. A crystalline solid has a precise melting temperature because each atom or molecule of the same type is held in place with the same forces or energy. Amorphous solids or non-crystalline solids (or, sometimes, glasses) which lack an ordered internal structure and are randomly arranged. Substances that consist of large molecules, or a mixture of molecules whose movements are more restricted, often form amorphous solids. Amorphous material undergoes gradual softening, over a range of temperatures, due to the structural non-equivalence of the molecules. When an amorphous material is heated, the weakest intermolecular attractions break first. As the temperature is increased further, the stronger attractions are broken.
Unit Cell
The structure of a crystalline solid is best described by its simplest repeating unit, referred to as its unit cell. The unit cell consists of lattice points that represent the locations of atoms or ions. The entire structure then consists of this unit cell repeating in three dimensions, as illustrated in Figure 1.
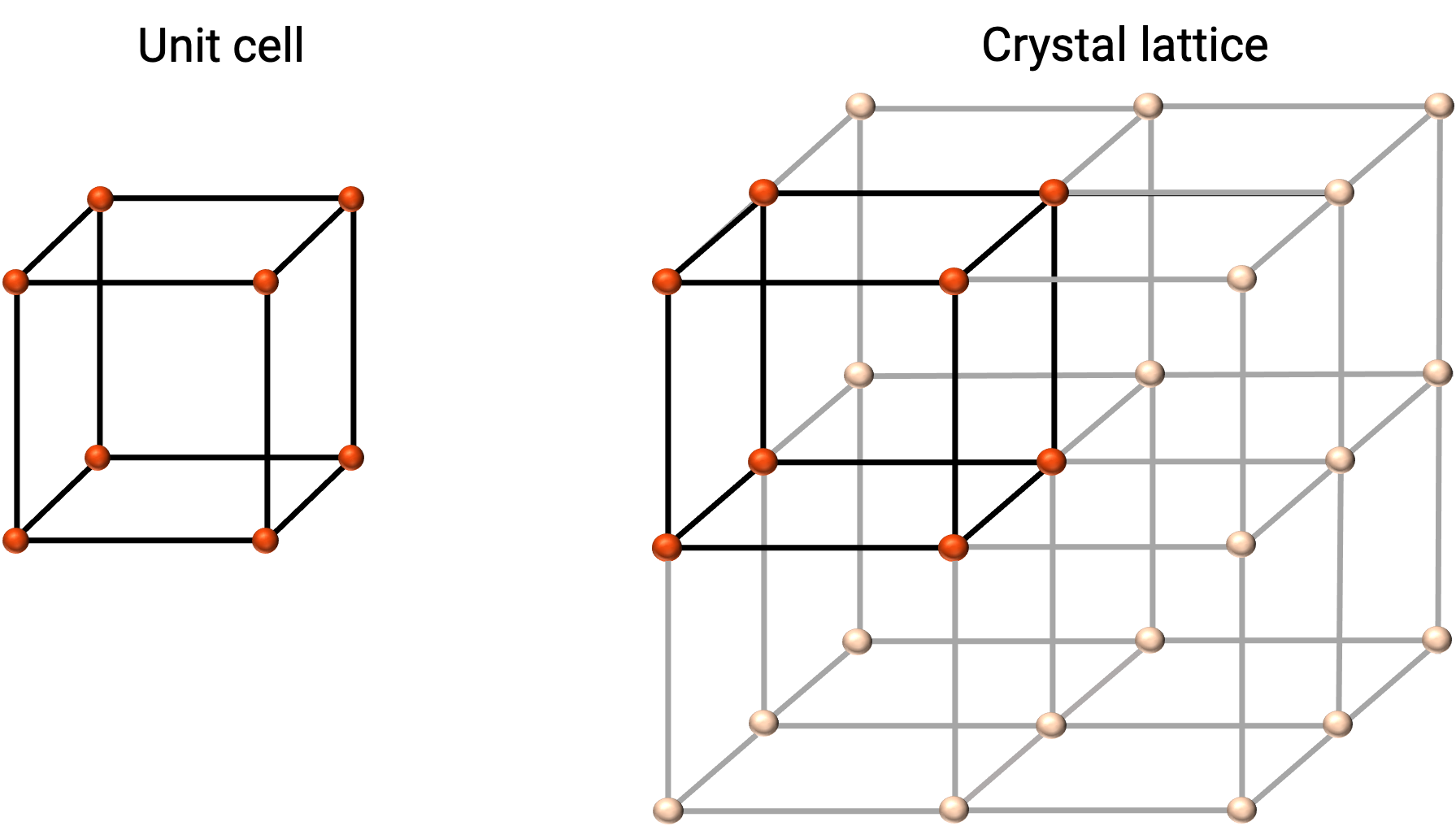
Figure 1. Unit cell and crystal lattice with lattice points indicated in red.
In general, a unit cell is defined by the lengths of three axes (a, b, and c) and the angles (α, β, and γ) between them as shown in Figure 2. The axes are defined as being the lengths between points in the space lattice.
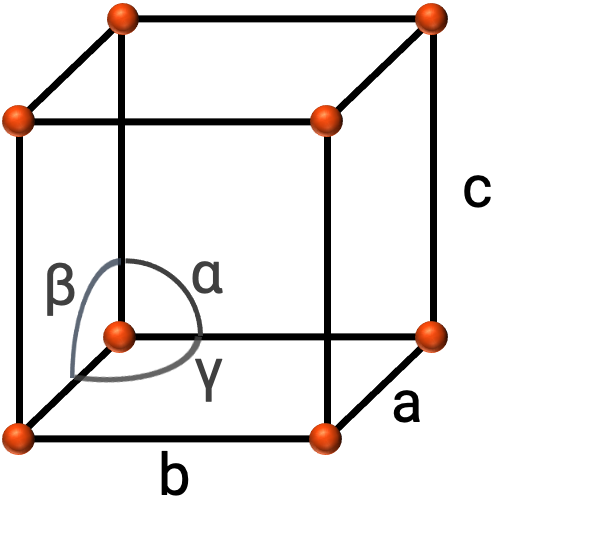
Figure 2. Unit cell is defined by its axes (a, b, and c), and angles (α, β, and γ)
There are seven different lattice systems, some of which have more than one type of lattice, for a total of fourteen different unit cells.
| Systems | Angles | Axes |
| Cubic | α = β = γ = 90° | a = b = c |
| Tetragonal | α = β = γ = 90° | a = b ≠ c |
| Orthorhombic | α= β = γ = 90° | a ≠ b ≠ c |
| Monoclinic | α = γ = 90°; β ≠ 90° | a ≠ b ≠ c |
| Triclinic | α ≠ β ≠ γ ≠ 90° | a ≠ b ≠ c |
This text is adapted from Openstax, Chemistry 2e, Section 10.6: Lattice Structures in Crystalline Solids.
From Chapter 11:

Now Playing
11.14 : Structures of Solids
Liquids, Solids, and Intermolecular Forces
12.6K Views

11.1 : Molecular Comparison of Gases, Liquids, and Solids
Liquids, Solids, and Intermolecular Forces
38.5K Views

11.2 : Intermolecular vs Intramolecular Forces
Liquids, Solids, and Intermolecular Forces
80.2K Views

11.3 : Intermolecular Forces
Liquids, Solids, and Intermolecular Forces
51.7K Views

11.4 : Comparing Intermolecular Forces: Melting Point, Boiling Point, and Miscibility
Liquids, Solids, and Intermolecular Forces
42.4K Views

11.5 : Surface Tension, Capillary Action, and Viscosity
Liquids, Solids, and Intermolecular Forces
26.7K Views

11.6 : Phase Transitions
Liquids, Solids, and Intermolecular Forces
17.9K Views

11.7 : Phase Transitions: Vaporization and Condensation
Liquids, Solids, and Intermolecular Forces
16.3K Views

11.8 : Vapor Pressure
Liquids, Solids, and Intermolecular Forces
32.8K Views

11.9 : Clausius-Clapeyron Equation
Liquids, Solids, and Intermolecular Forces
53.3K Views

11.10 : Phase Transitions: Melting and Freezing
Liquids, Solids, and Intermolecular Forces
11.8K Views

11.11 : Phase Transitions: Sublimation and Deposition
Liquids, Solids, and Intermolecular Forces
16.0K Views

11.12 : Heating and Cooling Curves
Liquids, Solids, and Intermolecular Forces
21.2K Views

11.13 : Phase Diagrams
Liquids, Solids, and Intermolecular Forces
36.4K Views

11.15 : Molecular and Ionic Solids
Liquids, Solids, and Intermolecular Forces
15.9K Views
See More
Copyright © 2025 MyJoVE Corporation. All rights reserved