The presence of a catalyst affects the rate of a chemical reaction. A catalyst is a substance that can increase the reaction rate without being consumed during the process. A basic comprehension of a catalysts’ role during chemical reactions can be understood from the concept of reaction mechanisms and energy diagrams.
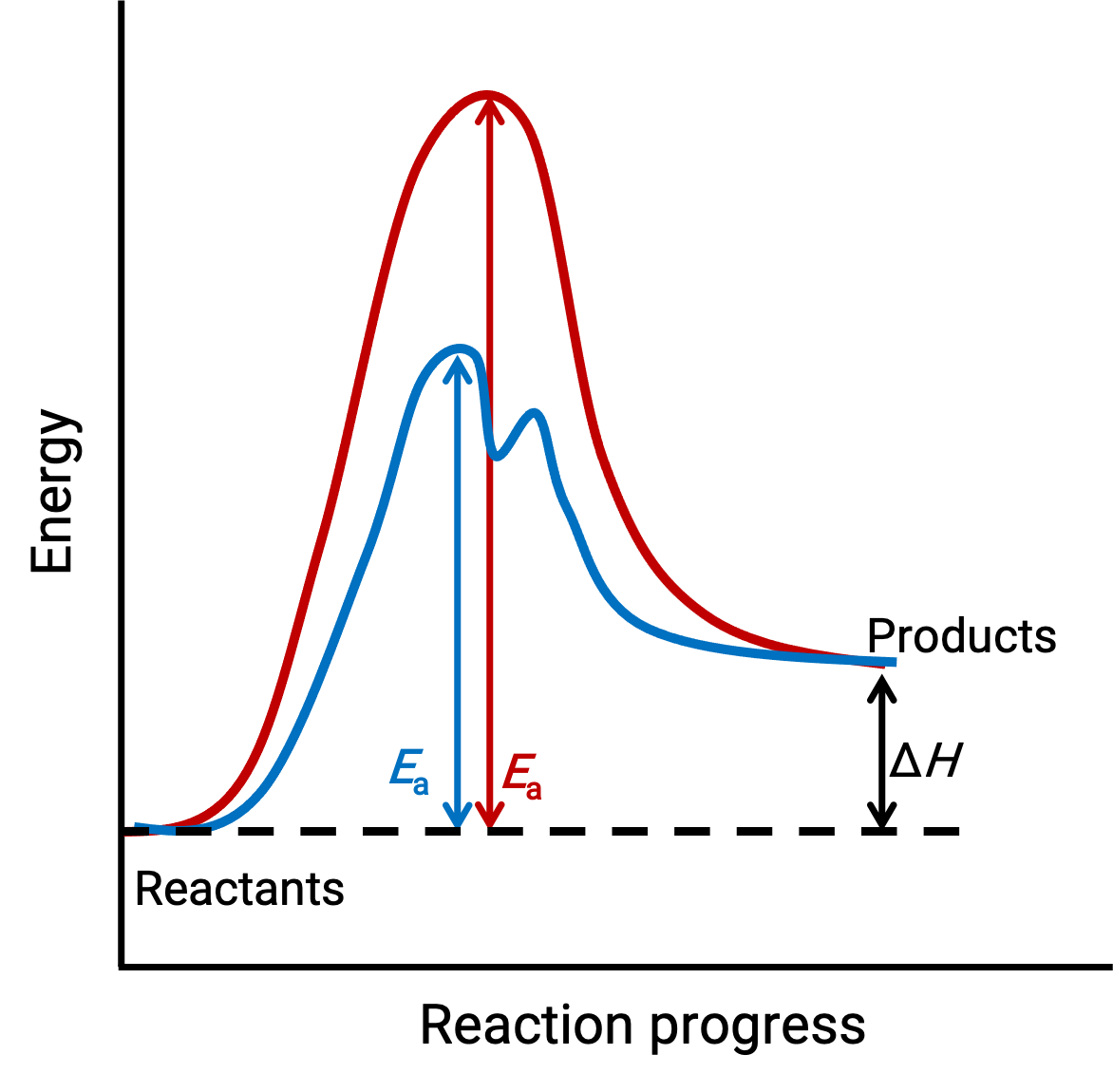
The illustrated image represents the reaction diagrams for an endothermic chemical process progressing in the absence (red curve) and presence (blue curve) of a catalyst.
Both curves represent the same overall reaction – they begin and end at the same energies. (In this case, products have more energy than reactants. Hence, the reaction is endothermic). However, their reaction mechanisms are different. The uncatalyzed reaction proceeds via a one-step mechanism (with only one observed transition state). In contrast, the catalyzed reaction follows a two-step mechanism (two transition states are observed) with notably lesser activation energy. This difference in the reaction pathways illustrates the catalyst’s role in providing an alternative reaction mechanism with lower activation energy, thereby accelerating reactions.
The catalyzed reaction mechanism does not need to involve a higher number of elementary steps than the uncatalyzed mechanism. However, it must provide an alternate reaction path whose rate-determining step is faster (with a lower Ea or activation energy).
A catalytic reaction may be categorized as homogeneous or heterogeneous, based on the physical states that catalysts and reactants exist during the catalytic process.
Homogeneous Catalysis
In homogeneous catalysis, the catalyst is present in the same phase as the reactants – solid, liquid, or gas. During the process, the catalyst interacts with the reactant to form an intermediate substance, which then decomposes or reacts with another reactant in one or more steps to regenerate the original catalyst and form the final product.
An example of homogeneous catalysis is the chemical process involving the decomposition of ozone occurring in the earth’s upper atmosphere. Ozone is a relatively unstable molecule that decomposes to yield diatomic oxygen. This decomposition reaction is consistent with the following two-step mechanism:
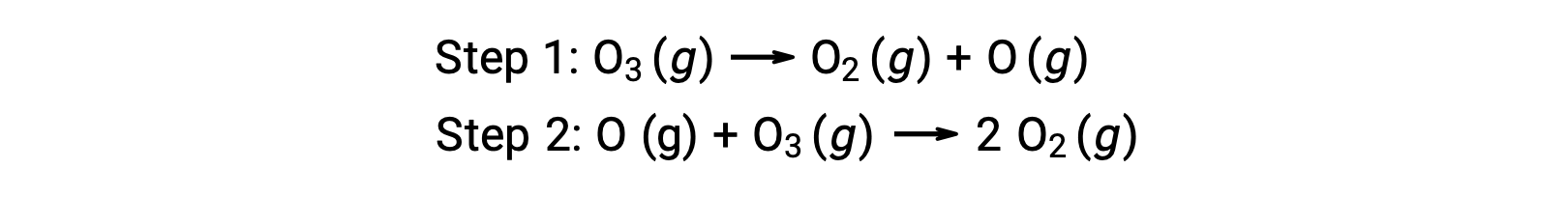
Many substances can catalyze the decomposition of ozone. For example, the nitric oxide–catalyzed decomposition of ozone is believed to occur via the following three-step mechanism:
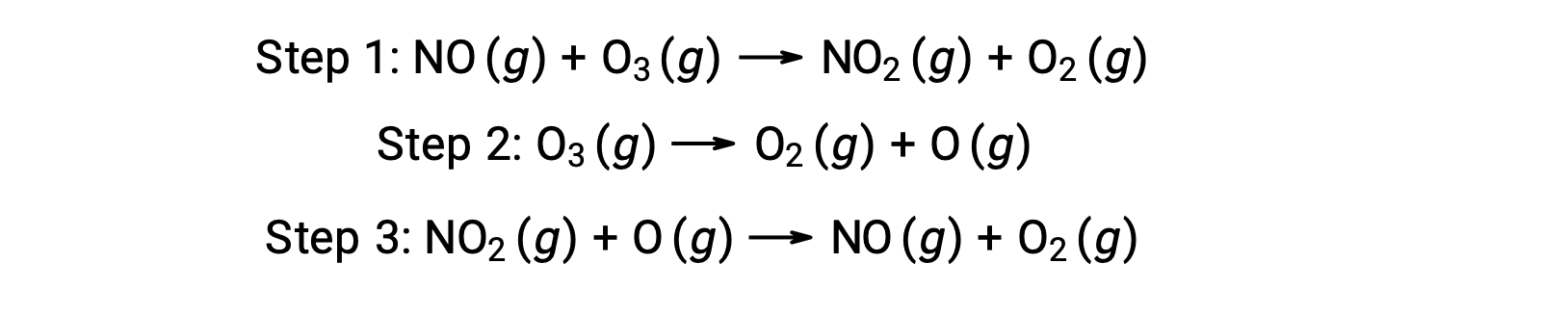
However, the overall reaction is the same for both the two-step uncatalyzed mechanism and the three-step NO-catalyzed mechanism:
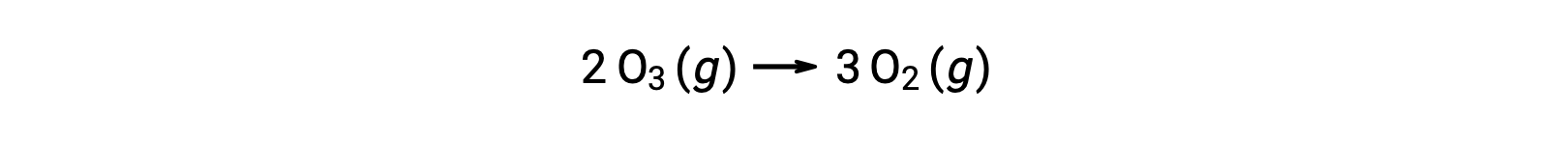
In the catalyzed reaction, notice that NO is a reactant in the first step of the mechanism and a product in the last step. This is another characteristic trait of a catalyst: Though it participates in the chemical reaction, it is not consumed by the reaction. Additionally, in this homogeneous catalysis, both the reactant and the catalyst exist in a gaseous phase.
Heterogeneous Catalysis
In heterogeneous catalysis, the catalyst is present in a different phase (usually a solid) than the reactants. Such catalysts generally function by furnishing an active surface upon which a reaction can occur. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the catalyst's surface rather than within the gas or liquid phase.
Heterogeneous catalysis typically involves the following processes:
- Adsorption of the reactant(s) on the surface of the catalyst
- Activation of the adsorbed reactant(s)
- The reaction of the adsorbed reactant(s)
- Desorption of product(s) from the surface of the catalyst
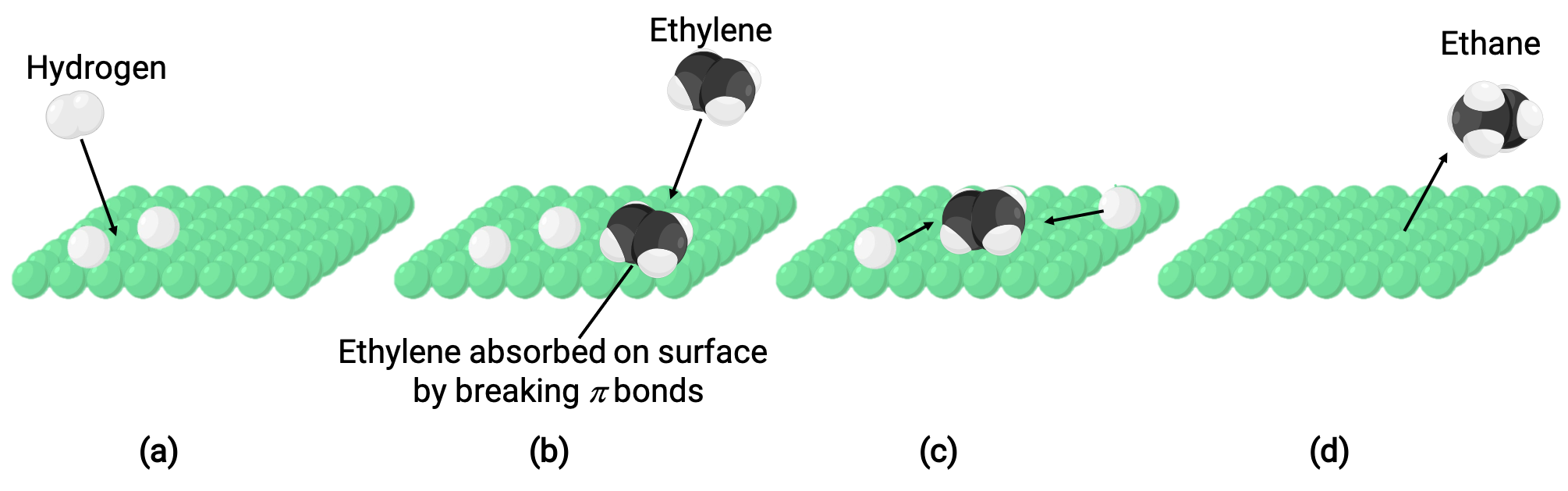
The illustrated image represents the reaction mechanism involving the heterogeneous catalysis of ethene and hydrogen gas on a solid nickel surface, forming ethane gas (C2H4 + H2 ⟶ C2H6):
(a) Hydrogen adsorbs on the nickel surface. During the process, hydrogen-hydrogen bonds are broken to form nickel-hydrogen bonds.
(b) Ethene also adsorbs on the nickel surface by breaking the carbon-carbon pi-bond and forming nickel–carbon bonds.
(c) Hydrogen atoms diffuse across the surface and form new carbon-hydrogen bonds when they collide to form ethane (C2H6).
(d) Ethane molecules desorb from the nickel surface.
Heterogeneous catalysis is used to industrially manufacture chemical products such as ammonia, nitric acid, sulfuric acid, and methanol. Heterogeneous catalysts are also used in the catalytic converters found on most gasoline-powered automobiles.
This text is adapted from Openstax, Chemistry 2e, Section 12.7: Catalysis.
From Chapter 13:

Now Playing
13.11 : Catalysis
Chemical Kinetics
25.6K Views

13.1 : Reaction Rate
Chemical Kinetics
48.7K Views

13.2 : Measuring Reaction Rates
Chemical Kinetics
22.8K Views

13.3 : Concentration and Rate Law
Chemical Kinetics
28.3K Views

13.4 : Determining Order of Reaction
Chemical Kinetics
52.7K Views

13.5 : The Integrated Rate Law: The Dependence of Concentration on Time
Chemical Kinetics
32.7K Views

13.6 : Half-life of a Reaction
Chemical Kinetics
32.7K Views

13.7 : Temperature Dependence on Reaction Rate
Chemical Kinetics
79.5K Views

13.8 : Arrhenius Plots
Chemical Kinetics
36.2K Views

13.9 : Reaction Mechanisms
Chemical Kinetics
24.2K Views

13.10 : Rate-Determining Steps
Chemical Kinetics
30.5K Views

13.12 : Enzymes
Chemical Kinetics
79.1K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved