Movement-Related Changes in Cortico-Pallidal Coupling Revealed by Simultaneous Intracranial and Magnetoencephalography Recordings in Dystonia Patients
Not Published
In This Article
Summary
Here, we describe how to combine simultaneous subcortical local field potential recordings and whole-head magnetoencephalography in patients with dystonia during the performance of a Stop-signal task. This method allows for the investigation of task-specific changes in network connectivity through changes in coherence.
Abstract
Primary dystonia is a movement disorder characterized by involuntary twisting movements and abnormal postures. It has been hypothesized that the pathophysiology of dystonia may arise from disturbed oscillatory connectivity between distant brain regions.An experimental setup of simultaneous local field potential (LFP) recordings from deep brain nuclei (here, globus pallidus internus) and whole-head magnetoencephalography (MEG) enables the investigation of cortico-subcortical connectivity patterns in patients with dystonia who underwent implantation of deep brain stimulation (DBS) electrodes. Our group previously described resting connectivity in three spatially distinct and frequency-specific cortico-pallidal networks: pallido-temporal coherence in the theta band (4-8 Hz), pallido-cerebellar coherence in the alpha band (7-13 Hz) and pallido-sensorimotor coherence in the beta band (13-30 Hz). The experimental approach also allows for investigation of task-specific changes in coherence between cortical and subcortical structures during motor processing. The methodology can easily be extended to emotional or cognitive processing, thereby opening a wide window for new research questions. Here, we demonstrate an investigation of pallido-sensorimotor beta band coherence during movement in a single illustrative patient.
Introduction
Patients with dystonia suffer from sustained or intermittent muscle contractions and abnormal posturing, sometimes accompanied by tremor1. Although it is suggested that dystonia is most likely associated with basal ganglia dysfunction2, abnormal brain activity has recently been identified in a wider network of regions including the cortex, cerebellum, brainstem and spinal cord3. While first line therapy for focal dystonia remains chemical denervation with botulinum toxin, pallidal deep brain stimulation (DBS) is a successful therapeutic option for otherwise refractory or complex cases4,5.
Externalization of DBS electrodes for clinical test stimulation before connection to the pulse generator gives the unique opportunity of recording electrophysiological activity from the target area. Such local field potential (LFP) recordings of the globus pallidus internus (GPi) revealed a characteristic increase in local low frequency (4 - 8 Hz) activity in dystonia6,7. Given that pallidal low-frequency oscillations temporally lead dystonic muscle activity, it has been suggested that they may play a causal role in dystonic symptom generation8. However, it is unclear whether the increased synchronization in GPi is accompanied by altered functional connectivity between brain areas.
Recently, a few research centers have started to use subcortical LFP recordings via DBS electrodes in combination with simultaneous whole-head magnetoencephalography (MEG) to study the electrophysiology of functional connections within the cortico-basal ganglia network in patients undergoing DBS for movement disorders9,10,11,12,13. Functional connectivity can be assessed through coherence of two signals, which quantifies their degree of oscillatory synchronization and can be interpreted as interaction or communication between brain areas14. Using this technique, we have previously identified a frequency-specific and spatially-distinct cortico-pallidal source of beta band coherence (13-30 Hz) over sensorimotor areas in dystonia patients at rest, as well as a pallido-temporal source of theta band (4-8 Hz) coherence and a pallido-cerebellar source of alpha band (7-13 Hz) coherence9. To investigate the role of sensorimotor beta coherence for movement generation, we used simultaneous GPi-LFP and MEG recordings in patients with dystonia while they performed a Stop-signal task and focused on movement-related changes in coherence13. In the following protocol, we describe this experimental approach in detail for one patient (female, age 48) with generalized dystonia with prominent lower body involvement who underwent implantation of pallidal DBS electrodes at the neurosurgery department of Charité - Universitätsmedizin Berlin.
Protocol
The study was approved by the local ethics committee of the Charité - University Medicine Berlin, Campus Virchow Klinikum, and was conducted in accordance with the declaration of Helsinki.
1. Patient Selection and Preparation of Experiment
- Identify appropriate subjects for study: subjects who have been referred for pallidal DBS because of focal, segmental or generalized dystonia. Exclude patients with 1) severe phasic head movements or head tremor to avoid movement artifacts in the MEG recordings, 2) implanted metallic medical devices to avoid artifacts in the MEG recordings, or 3) an inability to give informed consent.
- Conduct the experiment in the time interval between surgical implantation of the DBS electrodes and the implantation of the pulse generator device 5-7 days later.
NOTE: The DBS electrodes are externalized during this time and can therefore be used for intracerebral recordings. - Inform the patient about the possibility to partake in a scientific experiment and explain the experimental procedure the day after the DBS-electrode implantation. Hand over an information sheet about the experiment. Sign informed consent forms at least 24 hours later to ensure that the patient has enough time to consider his/her participation.
NOTE: Participating in the experiment does not bring additional risks for the patient and does also not delay the onset of their treatment. - The day before recordings, train the subject with a block (100 trials) of the experimental paradigm. The presented results here are from a Stop-signal task (Figure 1):
- Have the subjects hold an MEG-compatible response box in each hand, of which one button is used. Use professional experiment presentation software using a custom-written script to present stimuli.
- Start each trial with a black fixation cross against a white background. After a variable delay of 4-6 s, present the Go-signal, a double arrow pointing towards the left or right. Instruct the subject to respond as quickly as possible by pressing the button with the index finger of the corresponding hand.
- In 33% of the trials, present a Stop-signal, a red box around the Go-signal, at a variable delay after the Go-signal. In this case, instruct the subject to withhold the button press and wait for the next trial.
- Update the Stop-signal delay value in a trial-by-trial fashion via a staircase procedure to ensure 50% performance accuracy. Increase the delay by 50 ms after a successful Stop trial, and decrease by 50 ms after a Stop trial in which the subject fails to inhibit their response.
- Display the Go-signals until a response has been made or for a maximum duration of 1 s.
- Proceed with the next trial until the end of the experimental block.
- For information on precise localizations of DBS electrodes within the pallidum, use the Lead-DBS toolbox: 1) fuse preoperative and postoperative magnetic resonance images (MRI) or computed tomography (CT) scans, 2) normalize to Montreal Neurological Institute (MNI) space, and 3) map electrode contacts to a normalized subcortical atlas (for detailed information see Horn and Kühn, 201515).
2. Preparation of LFP-MEG Setup
- Accompany the subject to the MEG facility in presence of a medical doctor.
- Upon arrival, ask the subject and the medical doctor to take off all removable metal objects.
- Put on medical gloves and carefully remove the bandage around the percutaneous wires on the subject's head. Clean the electrode wires and external connectors from blood and iodine residuals and apply disinfection spray on the externalized materials.
- Locate the nasion and left and right pre-auricular points and place the three head position coils there.
- Clean and abrade the skin (~2 cm) and apply contact gel above the eyes for placement of electro-oculography (EOG) electrodes at 2 cm above the right eye and 1 cm to the right of the right canthus. Proceed likewise on the first dorsal interosseous muscle of the left and right hand. Place one electromyography (EMG) electrode on the belly of the muscle between index finger and thumb, and another on the metacarpophalangeal joint of the index finger.
- Fix the head positions coils, EOG and EMG electrodes with bandage material.
- Guide the subject into the magnetically shielded room and gently place the subject into a supine position with the head inside the MEG helmet. Leave a medically trained professional in the magnetically shielded room during the experiment seated in the corner of the room at a distance of 2.5 m from the MEG helmet. The subject and the medically trained professional should wear clothing free of any metal.
- Instruct the subject to avoid movement other than the button presses on the non-magnetic response boxes.
NOTE: These boxes are compatible with MEG, as they are made solely of glass fibers and plastics. - Connect the externalized DBS, EOG and EMG electrodes to an electroencephalography (EEG) amplifier integrated with the MEG. Connect the response boxes to the personal computer outside the shielded room running the experiment presentation software.
NOTE: The EEG amplifier used for recordings from the externalized DBS electrodes must be designed, safety tested, and validated to class BF (body floating). - Make sure the subject is in a comfortable position and feels at ease within the room as some subjects may suffer from claustrophobia. Emphasize that the subject may indicate a wish to stop the experiment at any time, for example when they feel very fatigued. In that case, terminate the recordings.
- Exit and close the magnetically shielded room. Give instructions to the subject through an integrated intercommunication system.
3. Recording LFP and MEG Signals
- Start the MEG data acquisition software and switch on the amplifiers and the SQUID sensors of the MEG through the control software.
- Set the parameters in the data acquisition software for a continuous and synchronous recording of pallidal LFP, EOG, EMG, MEG, stimulus trigger, and response button signals. Settings of the EEG and MEG channels are a high-pass filter (in hardware) of 0.1 Hz and a low-pass filter of 250 Hz. Set sampling rate to 2 kHz.
- Use the MEG control software to activate the head position coils to measure the head position in the MEG helmet. Repeat this measurement between blocks to assess head movement.
- Record the LFP signals using a common reference connected to the lowermost contact of the left DBS electrode. Offline re-referencing yields bipolar signals (c.f. section 4)
- Run the presentation software. The visual stimuli are generated by a separate computer with the presentation software installed. This generates the timing and sequence of visual stimuli that are projected from outside the shielded room onto a translucent glass panel that is visible to the subject at 20 cm distance from the subject's head.
- Run the first recording, which is a three-minute rest recording that measures the outputs listed in step 3.2. Instruct the subject to keep their eyes open and to fixate their gaze at a fixation cross that is displayed on the glass panel. Measure the head position before and after the rest recording.
- Next, run the task-specific recording. Instruct the subject that this recording will require performing the task as previously trained (see step 1.4). Also, instruct the subject to keep their head and body as still as possible during the recording. Measure the head position before and after the task.
- Record during task participation in 2 blocks of 100 trials. Between blocks, propose rest time to the subject if needed.
4. Preprocessing of MEG and LFP Recordings
- Analyze recorded data sets in a numerical computing environment using custom scripts and functions from SPM12 (http://www.fil.ion.ucl.ac.uk/spm/)16, DAiSS (https://github.com/spm/DAiSS) and Fieldtrip-20170307 (http://www.ru.nl/neuroimaging/fieldtrip/)17 toolboxes.
NOTE: Mentioned toolboxes are recommendations. It is of course also possible to analyze the data with software and toolboxes of your choice. - Read the raw signals into the computing environment (function: spm_eeg_convert.m).
- Visualize all data to inspect signal quality and correct channel assignments (function: spm_eeg_review.m).
- Re-reference the LFP signals to bipolar montages by subtraction of recordings from adjacent contacts to limit effects of volume conduction. Include all contact pairs in further data analysis to avoid selection bias.
- Down-sample the data to 1 kHz (function: spm_eeg_downsample.m) and apply notch filters at 50 Hz and higher-order harmonics to eliminate power line noise with fifth order zero-phase Butterworth filters (function: spm_eeg_filter.m).
- Divide recordings into trials of 7 s length around movement onset (function: spm_eeg_epochs.m).
- Discard trials with LFP amplitude values exceeding 7 standard deviations around the mean, as they are likely to be artifacts.
- Consider a segment saturated when the differences between adjacent samples stays <5 fT for ≥10 consecutive samples. Exclude channels when >20% of the epochs are contaminated by these artifacts (function: spm_eeg_artefact.m). Note: The MEG system used here has a rather low dynamic range which results in intermittent saturations in some channels that are close to the wires.
- Visually inspect all data after artifact rejection to ensure sufficient control of artifacts (see 4.3).
- For characterization of movement-related changes in sensorimotor cortico-pallidal coherence, include only Go-trials with successful motor response as determined from the response button channels.
5. Characterization of LFP-MEG Coherence
- Compute coherence between bipolar GPi-LFP and MEG epoched data via Welch's method with 50% overlapping 250 ms Hanning windows18 using all available data (function: ms_cohere.m). Visualize results for the a-priori chosen beta frequency range (13-30 Hz) as a topography (function: ft_topoplotTFR.m).
- Ensure that for each LFP channel the five MEG sensors with largest coherence values are selected.
- Re-compute coherence spectra for a time interval before movement (-3 s to 1 s, defined as 'rest') and a time window during movement (-1 s to 1 s) using the selected LFP-MEG channel combinations.
- Average resulting coherence spectra for left- and right-hand conditions to show the difference between rest and movement time intervals for the LFP electrodes of one hemisphere (see Figure 2).
- Check whether the head localization measurements before and after the rest recording have been performed successfully. This is achieved if the goodness-of-fit is at least 98%. Create a head model based on the inner skull derived from the subjects MRI with marked fiducial points (using SPM).
- Use the DICS (dynamic imaging of coherent sources) beamforming method for identification of the underlying source of the beta coherence seen on sensor level in resting data (using DAiSS). Compute coherence values on a 3D grid in MNI space and use linear interpolation at the grid points to produce volumetric images with 2 mm resolution. Visualize the result in 3D (see Figure 3).
6. Statistics
- In case the experiment is repeated for additional subjects, perform paired-samples t-tests on the coherence spectra to test for significant changes in coherence between rest and movement13.
- Obtain group-level statistics for the source localization by comparing real and surrogate coherence images in a fixed-effect ANOVA with subject and side as additional factors9.
Results
The presented data here stems from a female patient, 48 years old, presenting with generalized dystonia (preoperative Burke van Marsden Dystonia Rating Score19 of 19) and a disease duration of 20 years. Results from this subject show lateralized coherence in the averaged beta band (13-30 Hz) for MEG channels overlying ipsilateral sensorimotor areas when averaged across the entire trial (-3 to +3 s around movement onset); see Figure 2, upper panel.
When assessing the entire coherence spectrum averaged across the selected LFP-MEG channel combinations, a distinct coherence peak can be identified at 18 Hz. This shows that sensorimotor cortico-pallidal coherence is specifically present in the beta band. During movement, coherence in the beta frequency range decreased compared to rest. This decrease is more accentuated in frequencies of the low-beta range (13-20 Hz); see Figure 2, lower panel.
For the same patient, the source of the beta band coherence at rest was localized using beamforming. A peak was found in the sensorimotor cortex, as seen in Figure 3.
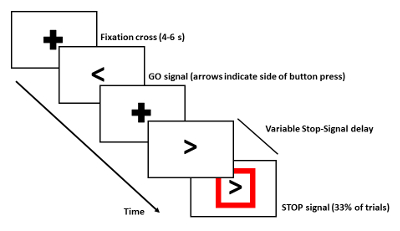
Figure 1: Scheme of Stop-signal Task. For detailed description see Protocol step 1.4. Only Go-trials with button presses were used to investigate movement-related changes in coherence between the left Gpiand cortex. Please click here to view a larger version of this figure.
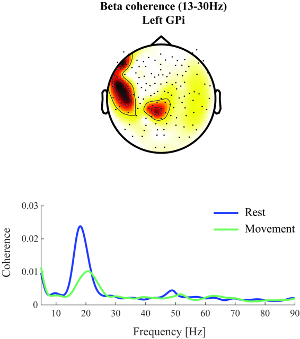
Figure 2: Beta band coherence. Upper panel: Beta band (13-30 Hz) coherence between the left GPi and cortex could be observed at rest for MEG channels overlying ipsilateral sensorimotor areas (nose at top, sensors indicated by black dots). Red colors indicate stronger coherence values. Lower panel: Beta band coherence was found to decrease during movement compared to rest. Shown is the coherence spectrum averaged across selected left-hemisphere LFP-MEG channel combinations and left- and right-hand movement conditions. The blue line indicates average coherence before movement onset and the green line coherence during movement (-1 to +1 s around movement onset). Please click here to view a larger version of this figure.
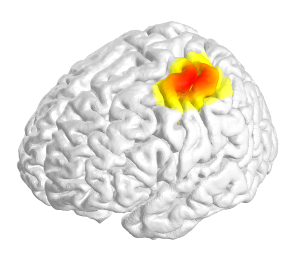
Figure 3: Dynamic Imaging of Coherent Sources (DICS) beamforming. DICS beamforming was used to localize the anatomical source of cortico-pallidal coherence for the same subject as presented in Figure 1. A spatially and spectrally focal peak of coherence was found in the ipsilateral sensorimotor cortex (red colors indicate higher coherence values). Please click here to view a larger version of this figure.
Discussion
In this article we show how to perform simultaneous subcortical LFP recordings and whole-head MEG for the identification of frequency- and task-related changes in functional connectivity.
The externalization of DBS-electrodes in a short post-operative interval gives the unique opportunity of recording subcortical local activity from the DBS-target structure. A major difficulty in the attempt of simultaneously recording from those externalized leads within the MEG may consist of the occurrence of high-amplitude artifacts originating from the percutaneous extension wires. However, Litvak et al. (2010)20 have shown that these artifacts can be suppressed by using beamforming methods.
Our experimental procedure is demonstrated with an example of a simple, visually cued reaction time task in dystonia patients with externalized pallidal DBS leads allowing for subcortical LFP recordings in parallel with whole-head MEG. Representative results of one subject revealed a movement-related decrease in cortico-pallidal coherence estimates in the beta frequency band that was most pronounced in the low-beta range and localized over ipsilateral sensorimotor areas.
We have recorded both the movement-related and the rest activity in a group of subjects (n=9) with various subtypes of dystonia (cervical, segmental and generalized). Across patients, cortico-pallidal beta-band coherence significantly decreased before and during movement. For the low-beta frequencies (13-21 Hz), this decrease correlated with subjects' reaction time. For detailed information, see van Wijk et al. (2017)13. The source reconstruction of cortico-pallidal beta band coherence in the resting state as reported here for a single subject is also consistent across patients, as reported in Neumann et al. (2015)9.
It has to be kept in mind that these results derive from a population cohort with severe movement disorders. Indeed, the invasive nature of intracerebral recordings does not allow the comparison of results to a healthy control group. Instead, the occurrence of similar electrophysiological activity patterns across different pathologies (e.g., Dystonia and Parkinson's disease) may suggest the generalizability of certain phenomena to the physiological brain. Also, subjects may be tired in the post-operative interval and therefore have difficulties in performing experimental tasks that are cognitively very demanding. The willingness and patience of the subject are a critical factor in the protocol to which the investigator should be attentive.
Another limitation when reporting results from human brain recordings is the presumption that the recorded signal stems from a specific brain structure which cannot be verified histologically. Therefore, one can only rely on indirect verification of the electrode locations. Intraoperatively, multi-unit activity can be recorded that displays a structure-specific activity pattern. Clinically effective macrostimulation provides additional evidence for correct electrode placement. Post-operatively, the toolbox "Lead-DBS" (http://www.lead-dbs.org/) permits localization of DBS-electrodes by fusing pre- and post-operative images before normalizing to MNI space, which allows for comparison of electrode placement to subcortical atlases.
Those limitations and critical steps kept in mind, the combination of intracerebral recordings with whole-head MEG covers a very large part of the cortico-subcortical network. MEG is especially well suited for this experimental set-up when compared to electroencephalography (EEG), as it lacks any contact to the skin. Given that recordings can be realized only in a brief post-operative interval, patients still present with wounds and bandages which makes an EEG with equal spatial resolution to a 125 channel MEG very difficult to obtain. In addition, MEG signals are less disturbed by skull holes compared to EEG signals21, and therefore require less detailed head models for accurate source localization.
Summing up, we showed how cortico-pallidal beta coherence decreases during movement in an example subject. We described how to study functional connectivity between subcortical and cortical structures using simultaneous LFP and MEG recordings in patients with implanted DBS electrodes. LFP-MEG recordings from subjects with different medical conditions are very valuable for our understanding of both pathological and physiological functional connectivity between distant brain regions and its specific changes related to motor, cognitive or emotional tasks. Moreover, future developments may allow LFP-MEG recordings during deep brain stimulation22, which would shed light on the still poorly understood mechanisms underlying the therapeutic success of deep brain stimulation.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors want to thank the German Research Foundation (DFG) for supporting this work with a grant for clinical research group "KFO247".
Materials
| Name | Company | Catalog Number | Comments |
| Magnetoencephalogram/ MEG Vision | formerly Yokogawa, now Ricoh systems | not released yet | Yokogawa transferred the MEG business to Ricoh Company, Ltd. on 1st April, 2016. |
| Deep Brain Stimulation Macroelectrodes and percutaneous extension wires | Medtronic | DBS 3387 Lead Kit for Deep brain stimulation | www.medtronic.com |
| Matlab R2015a | Mathworks | Online | https://de.mathworks.com/store |
| Presentation Software | Neurobehavioral Systems | Online | http://www.neurobs.com/menu_licensing/prices |
| Supervisc EEG Gel | MedCat GmbH | V16 | supplies@medcat.de |
| Medical gloves | Vinyl 2000 PF | MT-2001-XL |
References
- Albanese, A., et al. Phenomenology and classification of dystonia: a consensus update. Mov. Disord. 28, 863-873 (2013).
- Hallett, M., Riederer, P., Reichmann, H., Youdim, M. B. H., Gerlach, M. . Parkinson's Disease and Related Disorders. , 485-488 (2006).
- Hendrix, C. M., Vitek, J. L. Toward a network model of dystonia. Ann. N. Y. Acad. Sci. 1265, 46-55 (2012).
- Kupsch, A., et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N. Engl. J. Med. 355, 1978-1990 (2006).
- Volkmann, J., et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol. 11, 1029-1038 (2012).
- Silberstein, P., et al. Patterning of globus pallidus local field potentials differs between Parkinson's disease and dystonia. Brain. 126, 2597 (2003).
- Liu, X., et al. The sensory and motor representation of synchronized oscillations in the globus pallidus in patients with primary dystonia. Brain. 131, 1562 (2008).
- Sharott, A., et al. Is the synchronization between pallidal and muscle activity in primary dystonia due to peripheral afferance or a motor drive. Brain. 131, 473 (2008).
- Neumann, W., et al. Cortico-pallidal oscillatory connectivity in patients with dystonia. Brain. 138, 1894-1906 (2015).
- Litvak, V., et al. Movement-related changes in local and long-range synchronization in parkinson 's disease revealed by simultaneous magnetoencephalography and intracranial recordings. J. Neurosci. 32, 10541-10553 (2012).
- Hirschmann, J., et al. Differential modulation of STN-cortical and cortico-muscular coherence by movement and levodopa in Parkinson 's disease. Neuroimage. 68, 203-213 (2013).
- Litvak, V., et al. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain. 134, 359 (2011).
- van Wijk, B. C. M., et al. Low-beta cortico-pallidal coherence decreases during movement and correlates with overall reaction time. Neuroimage. 159, 1-8 (2017).
- Fries, P. Rhythms for cognition: Communication through coherence. Neuron. 88, 220-235 (2015).
- Horn, A., Kühn, A. A. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 107, 127-135 (2015).
- Litvak, V., et al. EEG and MEG data analysis in SPM8. Comput. Intell. Neurosci. 2011, (2011).
- Oostenveld, R., Fries, P., Maris, E., Schoffelen, J. -. M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 156869 (2011).
- Welch, P. Fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacust. 15, 70-73 (1967).
- Comella, C. L., Leurgans, S., Wuu, J., Stebbins, G. T. Rating scales for dystoni A multicenter assessment. Mov. Disord. 18, 303-312 (2003).
- Litvak, V., et al. Optimized beamforming for simultaneous MEG and intracranial local field potential recordings in deep brain stimulation patients. Neuroimage. 50, 1578-1588 (2010).
- Lau, S., Flemming, L., Haueisen, J. Magnetoencephalography signals are influenced by skull defects. Clin. Neurophysiol. 125, 1653-1662 (2014).
- Oswal, A., et al. Analysis of simultaneous MEG and intracranial LFP recordings during Deep Brain Stimulation: A protocol and experimental validation. J. Neurosci. Methods. 261, 29-46 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved