The Efficacy of a Porcine Collagen Matrix in Keratinized Tissue Augmentation: A 6-Month Follow-up Study
Not Published
In This Article
Summary
The aim of this study was to evaluate the long-term stability of the results achieved in peri implant tissue grafting by means of a collagen matrix.
Abstract
When keratinized tissue width around dental implants is poorly represented, the clinician could resort to autogenous soft tissue grafting. Autogenous soft tissue grafting procedures are usually associated with a certain degree of morbidity. Collagen matrices could be used as an alternative to reduce morbidity and intra-operatory times. The aim of this study was to evaluate the effectiveness of a xenogeneic collagen matrix when used as soft tissue grafting in the peri-implant area. Vestibuloplasty and keratinized tissue reconstruction around dental implants were performed in 15 patients using a porcine collagen matrix as the graft substitute. Then the obtained keratinized tissues were measured and evaluated after 6 months, 1 year, 4 years, and 5 years. The average gain of keratinized tissue was 5.7 mm. After 6 months, a resorption of 37% was observed. Secondary outcomes like hemostasis and morbidity were recorded as well. No complications were observed and minimal pain was reported in all the subjects. No bleeding or adverse reaction occurred in any patients. The present study suggests how the porcine collagen matrix can be used in keratinized tissue augmentation, thus reducing morbidity and the need of a second surgical site with limited tissue availability.
Introduction
The aim of the present research is to evaluate the efficacy in achieving keratinized tissue augmentation of a collagen matrix in a standard apically positioned flap (APF) procedure. Today, it is widely recognized that a sufficient amount of keratinized tissue is more than desirable around both teeth and dental implants: this type of tissue prevents gingival recession in association with the presence of plaque 1.
A surgical technique based on an APF for vestibuloplasty associated with grafting materials (whether autologous or not) is considered the gold standard for soft tissue augmentation 2. In the past few years collagen matrices have been studied as a valid substitute for free gingival grafts, in particular the porcine collagen matrix 3,4.
The xenogeneic porcine collagen matrix used in this study is a class III medical device according to the Medical Device Directive 93/42 (EEC, European economic community, definitions: 1.1: long-term implant; 1.2: implantable; 8: resorbable and 17: porcine origin). It consists of a unique structure composed by two functional layers: a cell occlusive layer with a smooth surface made of collagen fibers in a compact texture, and an underlying porous face, specifically reconstituted from collagen fibers. The collagen is processed to favor immediate blood clot stabilization. This helps early vascularization phases5,6, leading to a soft-tissue cell ingrowth5 and excellent integration of the 3D collagen matrix with surrounding tissues5,6. Both the EU and US Food and Drug Administration cleared the product for regenerative therapy involving teeth and implants.
So far, a small number of studies have demonstrated that collagen matrices are reliable and comparable with free gingival grafts for keratinized tissue augmentation around both teeth or dental implants 7,9,10,11. Additional advantages are a lower patient morbidity due to the absence of a donor site and the high aesthetic value, matching the texture and color of the adjacent mucosa 8,11,12.
Protocol
The following protocol was approved by the institution's (Fondazione IRCCS Cà Granda) human research ethics committee
1. Patient Selection, Pre-Operative Instructions, and Preparation
- Consider both male and female patients, older than 18 years old, with deficient keratinized mucosa < 2 mm surrounding one or more dental implants as valid candidates.
NOTE: Exclude the following: smokers or diabetic patients; patients with a history of tumor in the head or neck area and/or radiotherapy; immunodeficiency; Sjogren syndrome, patients consuming bisphosphonates or drugs affecting the quality/quantity of soft and hard tissues in the oral cavity. - One week before surgery, send the patient's dental records to a dental laboratory to prepare a transparent acrylic stent that extends deep in the vestibule: this splint will be used after surgery.
- Mount a 105 mm macro lens on a Digital Single-Lens Reflex (DSLR) camera. Set the digital camera as following: diaphragm aperture 32, International Organization of Standardization (ISO) 200, exposition 1/125. Take digital pictures of the area of interest using intraoral mirrors.
- Instruct the patient to start mouth washes with 0.2% digluconate chlorehexidine twice a day, 1-day prior to surgery in order to disinfect the oral mucosa.
2. Surgery
- Receiving site preparation
- Infiltrate local anesthetic (articaine + adrenaline 1:100,000 in 1.8 mL cartridges, if no allergies are referred) with a syringe, equipped with a 30G 21 mm needle, in the area of interest.
- With a surgical scalpel (No. 15C blade), perform a split thickness (deep enough to reach the periosteum) incision based on the length of the area in need of treatment.
NOTE: Make the incision in the residual keratinized mucosal band, in order to promote the invasion of keratinized epithelial cells in the collagen matrix; record the time. - With the same surgical scalpel (No. 15C blade), perform two release split thickness incisions (deep enough to reach the periosteum), mesial and distal to the first one.
- Raise a split-thickness flap: while helping with surgical pliers and by using a #7 Goldman-Fox knife or a surgical scalpel (No. 15C blade), dissect the epidermal and connective tissue from the underlying periosteum.
- With the same instruments, carefully dissect the muscle fibers and reduce them to the depth of the vestibule, to maintain results over time.
- If present, remove fat tissue using a sharp surgical scissors to facilitate and achieve better healing.
- Suture the split-thickness flap, by means of a 5/0 nylon suture and a Castroviejo needle-holder, to the surrounding mucosa and periosteum, with separate stiches.
- Measure the length (between the two release incisions) and depth (to where the flap has been dissected apically) of the denuded area with a 15-mm North Carolina periodontal probe.
- Take digital photographs (as in step 1.3).
- Collagen matrix manipulation and application
- Open the sterile box of collagen matrix.
- With sharp surgical scissors, cut and shape the collagen matrix based on the measurements of the denuded area.
- Apply the collagen matrix: the porous layer has to be in contact with the underlying periosteum, while the smooth surface will face the vestibule.
- Suture the matrix with 5/0 nylon sutures to the surrounding tissue, being careful to have the coronal and apical portion of the matrix in contact with keratinized tissue; record the time (Figure 1).
- Evaluate the post-operative bleeding and hemostatic effect of the matrix. To evaluate bleeding, monitor the wound for 10 min and observe if any bleeding develops; if not bleeding, the matrix has an excellent hemostatic effect.
- Take digital photographs (as in step 1.3).
- Acrylic splint application (Figure 2)
- Make sure that the acrylic splint extends to the depth of the newly created vestibule: this will prevent muscle fibers to relapse, thus losing the initial height gain. The splint must be in close contact with the vestibular fornix, exercising a gentle pressure on it (the mucosa must not be stretched).
- Make sure, visually, that the splint is not in contact with the wound (the transparent material will help). The splint must not compress the collagen matrix, or poor healing could occur.
- Take digital photographs (as in step 1.3).
- Show the patient how to position the splint and how to remove it. To position the splint, a slight pressure will fix it to the surrounding teeth, while to remove it, simply apply force in the opposite direction. Instruct the patient to keep the splint in position for 10 days after surgery and to remove it for eating and cleaning.
- Instruct the patient to irrigate with a 10-mL syringe, the area twice a day for 10 days with a 0.9% NaCl solution and 0.2% digluconate chlorhexidine.
3. Follow-up
- Visit the patient after 3 days post-surgery (Figure 3).
- Assess post-operative pain the Mankoski's pain scale13 using as a reference, and assess bleeding (ask the patient if any bleeding was noticed in the two days after surgery).
- After 10 days, remove the sutures and instruct the patient to no longer wear the splint. (Figure 4).
NOTE: Some sutures can be left in place to better measure the grafted area (if needed). - Evaluate healing after 2 weeks. Evaluate the new epithelization of the grafted area. Remove all the remaining sutures (Figure 5).
- 1-Month Follow-up: Assess healing by visual examination (Figure 6).
- 2-Month Follow-up: Assess healing by visual examination. Healing should be complete in 100% of patients (Figure 7).
- 6-Month Follow-up: Assess tissue and color integration. The tissue must be indistinguishable from the adjacent mucosa (Figure 8).
- Take digital photographs at each step (from steps 3.2 to 3.7).
Representative Results
3 male and 12 female patients participated in the study, with 4 surgeries in the maxilla and 11 in the mandible. Depending on the size of the receiving area, measurements were taken from 3 to 5 points mesial to distal. The matrix's average size was approximately 22 mm in length per 8 mm in height. The pre-operative keratinized tissue mean value was 4.1 mm, whilst the post-operative value was 9.4 mm, leading to an initial mean gain of 5.3 mm. 6 months after surgery, a mean loss of 2.2 mm, corresponding to 39.5% height reduction of the original gain, occurred with a final result of 7.3 mm (Figure 9).
After 1 month, healing was complete in 14 out of 15 patients. After 6 months, the new tissue appeared fully matured and indistinguishable from the surrounding tissue. All data were elaborated with the Analysis of Variance (ANOVA) repeated measurements method. Genetical pre-determination of keratinized tissue height was confirmed as it was assessed clinically that this value was never lower than the adjacent sites. According to the post-operative experience described by the patients, there was no bleeding, and the level of pain felt after surgery was very low to no pain at all (only 2 patients took a mild analgesic).
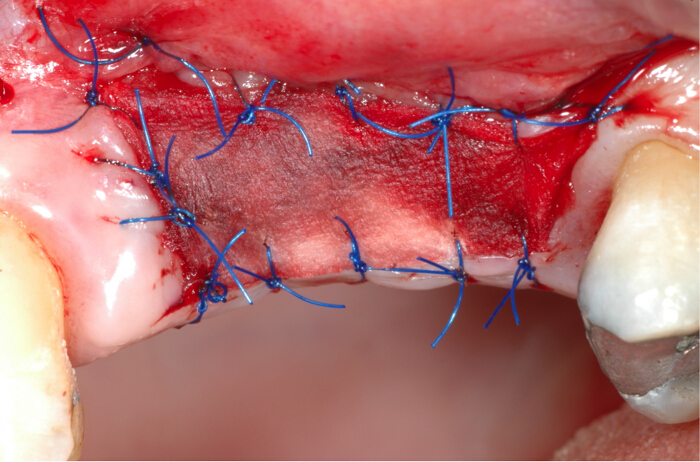
Figure 1. Collagen matrix placement in the soft tissues defect requiring keratinized gingiva augmentation. The matrix is designed and placed based on the defect area shape and extension. The collagen matrix is sutured to the surrounding tissues with 5/0 nylon stitches. Please click here to view a larger version of this figure.

Figure 2. Acrylic splint in position after surgery. The acrylic splint is placed over and not in contact with the collagen matrix to improve the new vestibule expansion. It will be kept for 10 days, and only removed during eating and cleaning. Please click here to view a larger version of this figure.

Figure 3. 3 days post-operative check. It is evident how the fibrin covers the entire graft. Please click here to view a larger version of this figure.

Figure 4. 10 days post-operative check. Healing is proceeding, and epithelization is starting. Please click here to view a larger version of this figure.

Figure 5. 14 days post-operative check. Few sutures are left in place to facilitate measurements, and epithelization is almost complete. Please click here to view a larger version of this figure.

Figure 6. Photograph taken at 1 month after surgery. Healing is complete and keratinized tissue is present. Please click here to view a larger version of this figure.

Figure 7. 2-Month follow-up check. A good quantity of fully matured keratinized tissue is visible. Please click here to view a larger version of this figure.

Figure 8. 6-Month follow-up check. The reduction of keratinized tissue is evident, but the residual amount is more than desirable for the final prosthetic rehabilitation. Please click here to view a larger version of this figure.

Figure 9. Graft shrinkage over time. It can be observed the 2.2 mm reduction of keratinized gingival in the 6-month period. Considering the original gain of 9.4 mm, the shrinkage showed a rate of 39.5%.
Discussion
The study's aim was to evaluate the efficacy of a xenogeneic collagen matrix, used as a soft tissue graft substitute in the reconstruction of an adequate amount (at least 2 mm) of keratinized peri-implant tissue. The main parameters taken into consideration were: shrinkage, re-epithelization, hemostatic effect, post-operative morbidity, and aesthetic outcome.
The outcome of the present study can be compared with the results of other papers found in literature reporting the use of non-autologous grafting in soft tissue reconstruction techniques. The protocol showed the efficacy of the collagen matrix in obtaining a new keratinized mucosa; however, it is important to focus on the critical steps of the technique: the manipulation phase and the suture phase. Despite the successful results, the device presented limits during the manipulation and designing due to its reduced thickness. For the same reason, despite the new level recorded of the keratinized tissue (7.3 mm), a reduced thickness compared to the areas surrounding the defects was observed clinically.
The suture phase required a significant amount of the procedure time due to the difficult manipulation and the higher number of sutures needed to stabilize the device. Sutures were placed with extreme precision all around the device's perimeter, in order to improve the contact area with the vascular bed and reduce possible movement in the early phases. The multiple single sutures around the entire border, the placement of the acrylic stent, and the post-operative instruction improved both the stability of the matrix and the healing during the first days, which represent the most critical phase of the healing period.
Wei and Laurell, in 2001, in a clinical and a histological study performed on 12 patients, compared the increment of adherent mucosa in two groups: the first composed of 6 patients receiving an autologous graft while the second, an allogeneic one 14,15. They observed an increment of adherent mucosa in both groups, but they noticed how the gain was very poor using the allogeneic graft. This was caused by an excessive shrinkage of the graft in the post-operative period. The histological analysis showed that a tissue very similar to scar tissue and incapable of inducing cellular differentiation, characterized all grafted sites.
Shrinkage and thickness are two of the most important parameters concerning soft tissue regenerative techniques. One of the most important issues when using the allogeneic material is the fast resorption 14,15, which leads to a disturbed healing. As for the connective tissue graft, the epithelialization of the acellular matrices starts from the surrounding tissue proliferation. To gain keratinized tissue, it is necessary to put the borders of the graft in contact with the host's keratinized mucosa 16. Sanz et al. in 2009 confirmed the reliability of xenogeneic collagen matrices, which provided predictable and satisfactory results. They evaluated, using a porcine derived collagen matrix, the efficacy in gaining keratinized tissue in comparison with an autologous graft. After 6 months, the data collected in both groups were nearly the same, showing an insignificant statistical variance (60% shrinkage for the autologous and 67% for the xenogeneic material) 17. The similarity of the results between the porcine and autograft groups suggest how this technique is not only comparable to the gold standard technique, but is also capable of reducing morbidity and intra-operative times, and avoiding the need of a donor site. This represents a significant point compared to the classic, existing method. In the present paper similar data were collected, even if the absence of a control group represented a limitation of the study.
The results presented here can be considered satisfactory for the set endpoints, according to the other authors. In all the mentioned studies here, including the present study, one factor is particularly highlighted concerning the post-operative course: the great decrease in morbidity16,17. The xenogeneic material is very easy to use and handle. The average time of surgery is about 30 minutes (excluding anesthesia)17. Furthermore, all grafted sites showed, once the healing was completed, an optimal integration with the surrounding area, and a good aesthetic profile, as compared to previous studies8,12,17.
The present study suggests how this type of collagen matrix can be used in patients needing a keratinized tissue augmentation surrounding dental implants, results in a great aesthetic outcome and causes very little pain. Future studies should focus on the thickness of the collagen matrices, its long-term stability, and its application in similar techniques in the intraoral district for keratinized augmentation.
Acknowledgements
The authors have no acknowledgments.
Materials
| Name | Company | Catalog Number | Comments |
| Mucograft | Gesitlich Pharma AG | Xenogenic porcine collagen matrix | |
| Ubistesin Articaine Hydrochloride 4% + epinephrine 1:200,000 | 3M | 15035 | Local Anesthetic |
| Curasept: Digluconate Chlorhexidine 0.2% | CURADEN HEALTHCARE S.p.A | Mouth wash | |
| 15c blade surgical scalpel | Henry-Schein / Krugg | 10 58330 | |
| Adson Surgical tissue pliers | Hu-Friedy | TP42 | |
| Goldman Fox n°7 | Hu-Friedy | KGF7CH | Surigcal knife |
| Straight Kelly scissors | Hu-Friedy | s2l | |
| Castroviejo | Hu-Friedy | NHCV | Needle-holder |
| Periodontal probe | Hu-Friedy | PCPUNC156 | 15mm North Carolina probe |
| 5/0 Ethilon Nylon Suture | Ethicon | 668h | Monofilament nylon suture |
| Protection splint | Technician Lab. | Methyl-methacrylate resin splint | |
| SPSS Statistics | IBM | Statistic analysis software | |
| Needle 30Gauge 21 mm | Heraeus | 09 85900 | |
| Nikon D90 DSLR camera | Nikon | Digital camera | |
| Sigma 105mm f2.8 | Sigma | Optical lens | |
| Intraoral mirror | Smart Europe | 32989 | Lateral intraoral mirror |
References
- Pranskunas, M., Poskevicius, L., Juodzbalys, G., Kubilius, R., Jimbo, R. Influence of Peri-Implant Soft Tissue Condition and Plaque Accumulation on Peri-Implantitis: a Systematic Review. J Oral Maxillofac Res. 9 (3), 7 (2016).
- Costello, B. J., Betts, N. J., Barber, H. D., Fonseca, R. J. Preprosthetic surgery for the edentulous patients. Dent Clin North Am. 40 (1), 19-38 (1996).
- Dragan, I. F., Hotlzman, L. P., Karimbux, N. Y., Morin, R. A., Bassir, S. H. Clinical Outcomes of Comparing Soft Tissue Alternatives to Free Gingival Graft: A Systematic Review and Meta-Analysis. J Evid Based Dent Pract. 17 (4), 370-380 (2017).
- Bassetti, R. G., Stähli, A., Bassetti, M. A., Sculean, A. Soft tissue augmentation around osseointegrated and uncovered dental implants: a systematic review. Clin Oral Investig. 21 (1), 53-70 (2017).
- Ghanaati, S., et al. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed Mater. 6 (1), 015010 (2011).
- Rocchietta, I., Schupbach, P., Ghezzi, C., Maschera, E., Simion, M. Soft tissue integration of a porcine collagen membrane: an experimental study in pigs. Int J Periodontics Restorative Dent. 32 (1), e34-e40 (2012).
- Vignoletti, F., et al. Healing of a xenogeneic collagen matrix for keratinized tissue augmentation. Clin Oral Implants Res. 26 (5), 545-552 (2015).
- Nevins, M., Nevins, M. L., Kim, S. W., Schupbach, P., Kim, D. M. The use of mucograft collagen matrix to augment the zone of keratinized tissue around teeth: a pilot study. Int J Periodontics Restorative Dent. 31 (4), 367-373 (2011).
- Vignoletti, F., et al. Clinical and histological healing of a new collagen matrix in combination with the coronally advanced flap for the treatment of Miller class-I recession defects: an experimental study in the minipig. J Clin Periodontol. 38 (9), 847-855 (2011).
- Jepsen, K., et al. Treatment of gingival recession defects with a coronally advanced flap and a xenogeneic collagen matrix: a multicenter randomized clinical trial. J Clin Periodontol. 40 (1), 82-89 (2013).
- Schmitt, C. M., et al. Vestibuloplasty: porcine collagen matrix versus free gingival graft: a clinical and histologic study. J Periodontol. 84 (7), 914-923 (2013).
- Sanz, M., Lorenzo, R., Aranda, J. J., Martin, C., Orsini, M. Clinical evaluation of a new collagen matrix (Mucograft prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: a randomized prospective clinical trial. J Clin Periodontol. 36 (10), 868-876 (2009).
- Mankoski, A. . Mankoski Pain Scale. , (1996).
- Wei, P. C., Laurell, L., Geivelis, M., Lingen, M. W., Maddalozzo, D. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 1. J Periodontol. 71 (8), 1297-1305 (2000).
- Wei, P. C., Laurell, L., Lingen, M. W., Geivelis, M. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 2. A histological comparative study. J Periodontol. 73 (3), 257-265 (2002).
- Imberman, M. Gingival augmentation with an acellular dermal matrix revisited: surgical technique for gingival grafting. Pract Proced Aesthet Dent. 19 (2), 123-128 (2007).
- Schmitt, C. M., et al. Vestibuloplasty: porcine collagen matrix versus free gingival graft: a clinical and histologic study. J Periodontol. 84 (7), 914-923 (2013).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved