Endotracheal Intubation via Tracheotomy and Subsequent Thoracotomy in Rats for Non-Survival Applications
In This Article
Summary
Here we introduce a standardized procedure for endotracheal intubation via tracheotomy followed by thoracotomy in rats, aimed at enhancing the precision and reproducibility of non-survival applications requiring invasive ventilation and exposure of thoracic organs in in vivo rat models.
Abstract
Endotracheal intubation and subsequent ventilation are often basic requirements for translational research in rat models for various interventions that require controlled or high ventilation pressures or access to the thoracic cavity and organs. Conventional endoorotracheal intubation using the anatomically existing route through the mouth is well suited for survival experiments. However, this procedure poses some challenges, including generally higher levels of the required experience and technical skill, more advanced equipment, and greater time effort with relevant intubation failure rates and complications such as tracheal perforation, temporary systemic hypooxygenation, and relevant aerial leakage.
This manuscript, therefore, presents a detailed step-by-step protocol for endotracheal intubation through tracheotomy in non-survival rat models when guaranteed intubation success, constant oxygenation levels, high ventilation pressures, or open thoracotomy are required.
The protocol emphasizes the importance of meticulous surgical technique to ensure consistent and reliable outcomes, especially for researchers who are inexperienced or lack routine in the technique of endoorotracheal intubation via direct laryngoscopy. This procedure is, therefore, expected to minimize animal suffering and unnecessary animal losses.
Introduction
In general, rodents are able to compensate for respiratory distress for much longer than patients would. They can remain cardiocirculatory stable and sufficient under spontaneous ventilation for much longer and during more invasive procedures, e.g., even through liver transplantation, which is known to be one of the procedures most stressful for cardiopulmonary circulation1,2,3.
However, endotracheal intubation is a basic requirement for translational research in rat models for various settings and interventions originating from a variety of biomedical fields and is essential in today's scientific landscape4,5,6,7,8,9,10,11. While the majority of experimental laboratory animal work performed in rats in general still does not require invasive ventilation12, there are certain experimental setups that require intubation and controlled ventilatory assistance. These experimental setups include any experiment requiring a secured airway, high ventilation pressures, and laparoscopy or direct access to the thoracic cavity and organs.
Especially in the case of required access to the thoracic cavity and organs, endotracheal intubation is imperative as the collapse of the lung will result in a fatal respiratory insufficiency once the negative pressure in the intrapleural space and the inspiration mechanism by the intercostal muscles and the diaphragm are lost13.
While there are many publications on methods for non-invasive endoorotracheal intubation in rodents14,15,16,17, there seems to be a lack of procedure protocols for invasive endotracheal intubation by tracheotomy. Despite the invasiveness of the latter, which only allows for application in non-survival animal models, there are great advantages of intubation via tracheotomy. These include a steeper learning curve, higher and more persistent success rates, less required technical equipment, and improved success monitoring opportunities18,19.
Intubation success is essential for outcome. While various false intubations, as well as repeated intubation attempts in patients, are clearly associated with adverse events and complications as serious as death20,21, such events are also deleterious in test animals. In the best case, they represent a strong confounder variable in the experiment, but they can also lead to the unnecessary loss of the animal. Therefore, it makes sense to increase intubation success rates at the cost of non-invasiveness if the experimental setup and strategy allow it.
This standardized protocol of intubation via surgical tracheotomy offers several advantages, including reduced variability in intubation success rates, minimized effects on the cardiorespiratory physiology parameters, and full investigational control and manipulation of parameters. It aids in providing a secured airway, especially for inexperienced researchers in the procedure of endotracheal intubation via direct laryngoscopy, and allows researchers to conduct non-survival studies with highly controlled conditions. We highlight key anatomical landmarks and provide insights into troubleshooting common challenges encountered during the procedure.
Protocol
All animal activities described here have been approved by the institutional animal care and use committee (IACUC) of the Baden-Württemberg Regional Council in Karlsruhe, Germany. Experimental animals were managed as per institutional standards and according to German laws for animal use and care and according to the directives of the European Community Council (2010/63/EU) as well as the ARRIVE guidelines and were conducted in accredited facilities. Male Spraque Dawley rats from Janvier Labs with an order weight of 400 g were used.
1. Procedure preparation (Figure 1)
- Prepare surgical preparation hooks by folding cannulas at an angle of 135° at 1 cm to the tip, connecting them to plastic perfusion tubes via Luer-lock, and applying tension using a surgical mosquito clamp (Figure 1A,B).
- Prepare a rodent surgical exposure apparatus.
NOTE: Besides the availability of commercial platforms, these can also easily be self-constructed cost-effectively, as in this study.- For this, use a 40 cm x 50 cm steel plate with a 4 mm thickness. Create several 8 mm holes using a steel drill at the desired location for subsequent tension points. Place the Y-shaped fixation rods in these locations.
- Place a heating pad on top of the steel plate of the surgical exposure apparatus in order to provide thermal support.
- Prepare the surgical preparation hooks with attached plastic tubes and surgical mosquito clamps to later apply tension to the tissue for surgical exposure.
- Remove the plug of a 10 mL plastic syringe with a male Luer-lock end and cut off the proximal end of the syringe. Insert the distal part into the ventilation bag as depicted and seal it airtight by covering it with a heat-shrinking tube and applying gentle heat using a laboratory torch (Figure 1D-F).
- Shorten and bevel different sizes of intravenous catheters and insert a guide wire from an arterial leader-cath set with the flexible wire end at the tip into the catheter size most appropriate for the respective animal size (presumably 14 G) for subsequent Seldinger technique22,23 during intubation (Figure 1G,H).
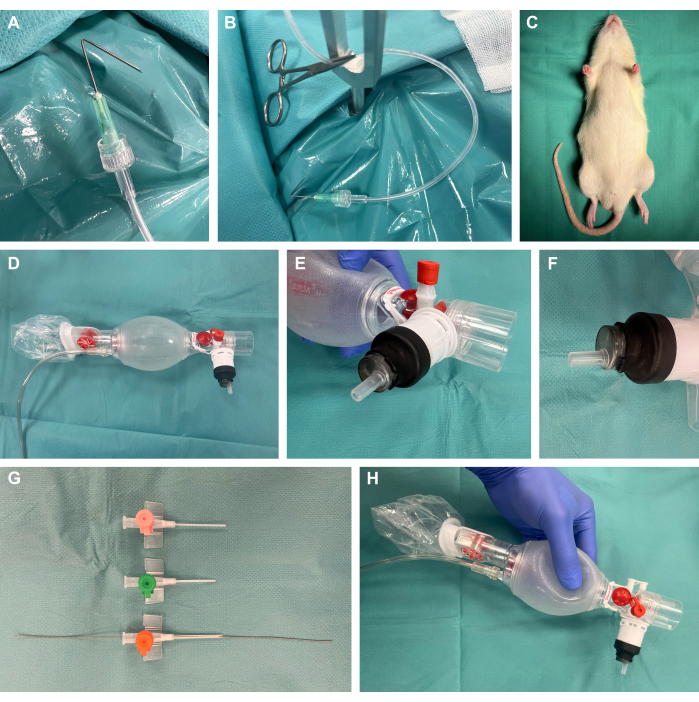
Figure 1: Experimental setup. (A) A folded cannula connected to a perfusion tube used as a surgical preparation hook. (B) Preparation hook with clamp positioned in a metal fixation rod. (C) Sedated animal prior to intervention. (D) Ventilation bag from neonatology. (E-F) Airtight mounting of a Luer-lock syringe onto the ventilation bag using a heat-shrinking tube. (G) Shortened and beveled intravenous catheters in different sizes with a guide wire from an arterial leader-cath set for the Seldinger technique. (H) Final custom-built construction. Please click here to view a larger version of this figure.
2. Anesthesia and analgesia
- Anesthetize the rat with the medication of choice.
NOTE: Here, anesthesia and analgesia were established using ketamine 10% (100 mg/mL), xylazine 2% (20 mg/mL), carprofen 50 mg/mL, and isoflurane. A 1:10 dilution of xylazine and carprofen were used as stock solutions for application.- Induce anesthesia by placing the animal in an induction box flooded with 100% oxygen at an inflow rate of 5 L/min complemented by 4% (v) of isoflurane using a vaporizer.
- After unconsciousness was achieved, remove the animal from the induction box and inject (i.p.) it with 100 mg/kg body weight ketamine and 4 mg/kg body weight xylazine.
NOTE: This corresponds to 40 mg of ketamine (0.4 mL of the original solution) and 1.6 mg of xylazine (0.08 mL of the original solution or 0.8 mL of the 1:10 solution) for a 400 mg animal. - Place the animal back into the isoflurane-flooded induction box until the effect of the i.p. injection sets in.
- Guarantee proper anesthetic depth by testing the lack of response of the rat to a firm toe pinch.
- Apply ophthalmic ointment to the eyes.
- Assess and maintain the depth of anesthesia during surgery.
- When narcotic effects seemed to diminish over time (about every hour), inject (s.c) the animal with an additional 50 mg/kg body weight ketamine and 1 mg/kg body weight xylazine. This corresponds to 20 mg of ketamine (0.2 mL of the original solution) and 0.4 mg of xylazine (0.2 mL of the 1:10 solution) for a 400 mg animal.
- Provide additional analgesia before thoracotomy with an s.c. injection of 5 mg/kg body weight carprofen. This corresponds to 2 mg of carprofen (0.04 mL of the original solution or 0.4 mL of the 1:10 solution) for a 400 mg animal.
3. Surgical preparation (Figure 2)
- Saturate the animal circulation with oxygen by offering inhalation of 100% oxygen via a transnasal neonatal mask (Figure 2A).
- After 5 min, switch from the transnasal neonatal mask to the construction of a neonatal ventilation bag with a male plastic syringe Luer-lock tip as described above and offer oxygen in overflow with the syringe tip close to the animal nasal tip (Figure 2B).
- Perform a median thoracocervical skin incision across the desired length and expose the surgical situs using the surgical preparation hooks (Figure 2C,D).
- Advance the preparation by blunt dissection with fine scissors through the cervical fascia. Expose the sternocleidomastoid and infrahyoidal muscle and lateralize the median infrahyoidal muscle to the right after left lateral membrane dissection (Figure 2E-G).
- Perform blunt dissection towards the trachea and tunneling of the trachea using overholt clamps (Figure 2H-I).
- Sling the trachea using a silicone vessel loop and double sling the distal trachea using a polyfilament suture for later endotracheal cannula fixation (Figure 2J-N).

Figure 2: Preparation of the trachea. (A) Inhalation of oxygen via mask. (B) Switch from mask to male Luer-lock syringe for oxygen application. (C) Thoracocervical skin incision. (D) Preparation through the cervical fascia. (E) exposure of the sternocleidomastoid muscle and the infrahyoidal muscle. (F) Lateralization of the median infrahyoidal muscle. (G) Blunt dissection towards the trachea. (H) tunneling of the trachea using overholt clamps. (I) Slinging of the trachea using a silicone vessel loop. (J-N) Caudal double slinging of the trachea using a poly filament suture for later endotracheal cannula fixation. Please click here to view a larger version of this figure.
4. Intubation procedure (Figure 3)
- Caudally lengthen the trachea using atraumatic pliers (Figure 3A,B).
- Perform a partial incision of the trachea of 180° of circumference using scissors (Figure 3C-F).
- Introduce a Seldinger guide wire with the flexible end into the trachea (Figure 3G-H) and subsequently introduce the modified intravenous catheter of appropriate girth into the trachea guided by the Seldinger wire (Figure 3I-N).
- Remove the Seldinger wire (Figure 3O), connect the Luer-lock tip of the tracheal catheter to the modified ventilation bag, and start gentle lung-protective manual ventilation with high frequency and low tidal volume (Figure 3P). Perform manual ventilation as long as median thoracotomy has not been performed and the lung is not in plain sight.
- Tie the previously placed locking suture with a sliding knot to avoid aerial leakage and accidental catheter removal (Figure 3Q-T).

Figure 3: Endotracheal intubation via tracheotomy. (A) Initial situation after preparation. (B) Caudal lengthening of the trachea using atraumatic pliers. (C-F) Partial incision of the trachea of 180° of circumference. (G,H) Introduction of a Seldinger guide wire into the trachea. (I-M) Introduction of the modified intravenous catheter into the trachea guided by the Seldinger wire. (N-O) Removal of the Seldinger wire. (P) Luer-lock connection of the tracheal catheter to the modified ventilation bag. (Q-T) Tying of the previously placed locking suture to avoid accidental catheter removal. Please click here to view a larger version of this figure.
5. Manual ventilation and median thoracotomy (Figure 4)
- Start the median thoracotomy from the xiphoid using blunt and stable material scissors (Figure 4A,B) and continue cranially through the sternum. Always pause the ventilation for a few seconds when advancing with the scissors substernally and when cutting in order to avoid trauma to the lung (Figure 4C-E).
- Gain thoracic exposure using surgical preparation hooks and remove mediastinal serosal membranes (Figure 4F-H).
- Continue manual ventilation with desired frequency and tidal volume and expand monitoring (e.g., establishing an intra-arterial blood pressure measurement, installing a central venous or pulmonary arterial catheter depending on the purpose or respective research question) (Figure 4I-L).

Figure 4: Manual ventilation and median thoracotomy. (A) initial situation after intubation. (B-E) Median thoracotomy starting from the xiphoid.(F-I) Thoracic exposure using surgical preparation hooks. (J) Final setup during ventilation. (K-L) the process of manual ventilation. Please click here to view a larger version of this figure.
6. Euthanasia
- Achieve euthanasia by abrupt sharp cardiectomy using scissors in the deeply anesthetized animal.
Representative Results
Endotracheal intubation via tracheotomy and subsequent thoracotomy was performed in 10 male rats (mean weight 405 ± 30 g) for non-survival applications. The procedure was performed by a second-year surgical resident. The success rate defined by survival over 20 min of intubation and ventilation was 100%. The mean duration of the preparation and intubation procedure from skin incision until fixation of the intubation tube was 6:55 ± 0:53 min (Table 1).
| Number of animals used | 10 |
| Body weight (mean and standard deviation) | 405 ± 30 g |
| Intubation success rate | 100% |
| Duration of procedure (mean and standard deviation) | 6:55 ± 0:53 min |
| Mean oxygen saturation | 96% |
| Minimum oxygen saturation | 92% |
Table 1: Representative results. Representative results of the protocol in 10 animals.
Saturation was monitored through a single-use self-stick pulse-oximetry probe on the hind leg and never fell below 92%. Spontaneous ventilation was contained until the incision of the diaphragm. All rats survived the 20 min required for the experimental measurements until the intentional euthanasia through abrupt sharp cardiectomy (Figure 5).

Figure 5: Peripheral oxygen saturation. Peripheral oxygen saturation measured over the whole duration of this procedure. Please click here to view a larger version of this figure.
Discussion
In the US alone, about 110 million rats and mice are used in animal experiments per year24. While no reliable statistics can be found, a relevant percentage of these will receive supported ventilation, especially in the fields of pulmonary and cardiac research as well as anesthesiology. Most of these animals receive non-invasive endoorotracheal intubation, which entails a number of challenges. These include slower learning curves, higher rates of intubation failure, greater numbers of reintubations, and associated respiratory complications as well as respiratory leakages25. Non-invasive intubation will, therefore, generally take more experienced research staff, more advanced technology such as fiber optics, capnometry, and mechanical ventilators, and greater financial and materialistic resources26. The whole aspect of intubation is, therefore, a meaningful confounder relevant in subsequent statistical analysis and interindividual heterogeneity of results. However, non-invasive endoorotracheal intubation is often the only feasible option in longitudinal experiment designs.
Alternatives are tracheostomy for required long-term tracheal access in longitudinal experiments or surgical tracheotomy for "single-use" in non-survival experiments. While protocols on the microsurgical technique for tracheostomy in rats are available27, there are currently no protocols for endotracheal intubation via tracheotomy in non-survival rat models.
The herein-described standardized procedure for endotracheal intubation via tracheotomy and subsequent thoracotomy in rats, therefore, represents a valuable method for researchers engaged in non-survival studies. This procedure requires neither expert personnel nor extensive training. It has an intubation success rate that is much higher than endoorotracheal intubation and allows for much more controlled procedural steps.
Limitations of the presented techniques mainly include the invasiveness of the procedure and, consecutively, the exclusive compatibility with non-survival experiments. Due to the large defects in skin and soft tissue, as well as the incisional tracheal fistula, extubation and recovery from this procedure are neither feasible nor recommended, especially when combined with thoracotomy.
When troubleshooting common challenges encountered during the procedure, we would like to draw attention to the following points and recommendations: one should prepare equipment and medication as extensively as possible. The experimenter should perform hemostatic control meticulously by fine preparation and using non-traumatic cotton swabs. The experimenter should prepare the trachea over a length of at least 0.5 cm in order to allow for sufficient mobility and use the silicone vessel loop in order to not lose the trachea after incision, as this can result in aspiration and suffocation when the trachea is lost in the tissue depth due to backdrop phenomenon. Only partially incise the trachea with one clear stroke of the scissors. Ideally, one should incise 180° of circumference. Below this might result in failure to intubate. Above this might result in snapping of the trachea with retraction of both ends into the depth of the tissue and subsequent suffocation. When tying the polyfilament suture to secure the intubation tube, a sliding knot should be used in order to ensure sufficient tightness to avoid air leakage or accidental tube dislocation while at the same time avoiding unintended closure of the tube. Leaving the Seldinger wire in place during suturing can avoid unintended closure. When performing manual ventilation using the ventilation bag, one should strictly avoid barotrauma of the lung by high-tidal volumes. Instead, one should rather aim for lung-protective, high-frequent, and low-tidal ventilation, especially as long as thoracotomy has not been performed and the lung volumes cannot be evaluated visually.
By offering a detailed and reproducible methodology, this protocol facilitates the standardization of experimental procedures, improving data reliability and comparability across studies. As a result, this method contributes to the refinement of scientific techniques in preclinical research, ultimately enhancing the translational relevance of findings in the fields of physiology, pharmacology, surgical and biomedical sciences.
Acknowledgements
The authors gratefully acknowledge the data storage service SDS@hd supported by the Ministry of Science, Research and the Arts Baden-Württemberg (MWK) and the German Research Foundation (DFG) through grant INST 35/1314-1 FUGG and INST 35/1503-1 FUGG. Furthermore, the authors gratefully acknowledge the support from the NCT (National Center for Tumor Diseases in Heidelberg, Germany) through its structured postdoc program and the Surgical Oncology program. We also acknowledge the support through state funds approved by the State Parliament of Baden-Württemberg for the Innovation Campus Health + Life Science Alliance Heidelberg Mannheim from the structured postdoc program for Alexander Studier-Fischer: Artificial Intelligence in Health (AIH) - A collaboration of DKFZ, EMBL, Heidelberg University, Heidelberg University Hospital, University Hospital Mannheim, Central Institute of Mental Health, and the Max Planck Institute for Medical Research. Furthermore, we acknowledge the support through the DKFZ Hector Cancer Institute at the University Medical Center Mannheim. For the publication fee, we acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding program "Open Access Publikationskoste" as well as by Heidelberg University.
Materials
| Name | Company | Catalog Number | Comments |
| Ambu Spur II Single-Use ventilation bag for neonates | Meier Medizintechnik | 335 102 000 | Ventilation bag |
| BD Microlance 3 cannula 20 G | BD (Beckton, Dickinson) | 301300 | Cannula |
| BD Microlance Discardit II 20 mL Syringe | BD (Beckton, Dickinson) | 300296 | Plastic syringe |
| Fixation rods | Legefirm | 500343896 | Tuning forks used as y-shaped metal fixation rods |
| Heat-shrinking tube | Sekesoer | RSG-400 | Heat-shrinking tube |
| Intravenous catheter | BD (Beckton, Dickinson) | 393230 | BD Venflon Pro Safety intravenous catheter 14 G; shortened using scissors; alternatively 16 G or 18 G can be used |
| Plastic perfusor tube | M. Schilling GmbH | S702NC150 | Connecting tube COEX 150 cm |
| Polyfilament suture | COVIDIEN | CL331 | Polyfilament surgical suture recommended with a strength of 1 to 2; needle can be removed |
| Royal Gardineer Heating Pad Size S, 20 Watt | Royal Gardineer | IP67 | Heating pad |
| Seldinger guide wire | VYGON | 115.798 | Metal guide wire from the arterial leadercath set |
| Silicone vessel loop tie | SERAG WIESSNER | SL26 | Silicone vessel loop tie 2.5 mm red |
| Spraque Dawley rats | Janvier Labs | Male rats weighing 400 grams | |
| Steel plate | Maschinenbau Feld GmbH | C010206 | Galvanized sheet plate, 40 x 50 cm, thickness 4.0 mm |
References
- Czigany, Z., et al. Limb remote ischemic conditioning of the recipient protects the liver in a rat model of arterialized orthotopic liver transplantation. PLoS One. 13 (4), e0195507 (2018).
- Czigány, Z., et al. Improving research practice in rat orthotopic and partial orthotopic liver transplantation: A review, recommendation, and publication guide. Eur Surg Res. 55 (1-2), 119-138 (2015).
- Nagai, K., Yagi, S., Uemoto, S., Tolba, R. H. Surgical procedures for a rat model of partial orthotopic liver transplantation with hepatic arterial reconstruction. J Vis Exp. 73, e4376 (2013).
- Jahshan, F., et al. A novel rat model for assessment of laryngotracheal injury following transoral intubation. Int J Pediatr Otorhinolaryngol. 113, 4-10 (2018).
- Lamoureux, L., Radhakrishnan, J., Gazmuri, R. J. A rat model of ventricular fibrillation and resuscitation by conventional closed-chest technique. J Vis Exp. 98, 52413 (2015).
- Wang, Z., et al. Autoinducer-2 of streptococcus mitis as a target molecule to inhibit pathogenic multi-species biofilm formation in vitro and in an endotracheal intubation rat model. Front Microbiol. 7, 88 (2016).
- Jahshan, F., et al. A novel rat model for tracheal mucosal damage assessment of following long term intubation. Int J Pediatr Otorhinolaryngol. 128, 109738 (2020).
- Rivard, A. L., et al. Rat intubation and ventilation for surgical research. J Invest Surg. 19 (4), 267-274 (2006).
- Na, N., Zhao, D. Q., Huang, Z. Y., Hong, L. Q. An improved method for rat intubation and thymectomy. Chin Med J (Engl). 124 (17), 2723-2727 (2011).
- Cicero, L., Fazzotta, S., Palumbo, V. D., Cassata, G., Lo Monte, A. I. Anesthesia protocols in laboratory animals used for scientific purposes. Acta Biomed. 89 (3), 337-342 (2018).
- Fuentes, J. M., et al. Videoendoscopic endotracheal intubation in the rat: A comprehensive rodent model of laparoscopic surgery. J Surg Res. 122 (2), 240-248 (2004).
- Bryda, E. C. The mighty mouse: The impact of rodents on advances in biomedical research. Mo Med. 110 (3), 207-211 (2013).
- Lazopoulos, A., et al. Open thoracotomy for pneumothorax. J Thorac Dis. 7 (S1), S50-S55 (2015).
- Rendell, V. R., Giamberardino, C., Li, J., Markert, M. L., Brennan, T. V. Complete thymectomy in adult rats with non-invasive endotracheal intubation. J Vis Exp. 94, 52152 (2014).
- Thomas, J. L., et al. Endotracheal intubation in mice via direct laryngoscopy using an otoscope. J Vis Exp. 86, 50269 (2014).
- Nelson, A. M., Nolan, K. E., Davis, I. C. Repeated orotracheal intubation in mice. J Vis Exp. 157, 60844 (2020).
- Das, S., Macdonald, K., Chang, H. Y., Mitzner, W. A simple method of mouse lung intubation. J Vis Exp. 73, e50318 (2013).
- Massick, D. D., et al. Quantification of the learning curve for percutaneous dilatational tracheotomy. Laryngoscope. 110 (2 Pt 1), 222-228 (2000).
- Giugliano, G., et al. Learning curve for translaryngeal tracheotomy in head and neck surgery. Laryngoscope. 111 (4 Pt 1), 628-633 (2001).
- Mort, T. C. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesth Analg. 99 (2), 607-613 (2004).
- Hasegawa, K., et al. Association between repeated intubation attempts and adverse events in emergency departments: An analysis of a multicenter prospective observational study. Ann Emerg Med. 60 (6), 749-754.e742 (2012).
- Titu, I. M., Delaca, G. B., Teterea, F., Ciulic, S. A., Palade, E. Percutaneous tracheostomy using the Seldinger technique. Multimed Man Cardiothorac Surg. 2023, (2023).
- Garry, B. P., Bivens, H. E. The Seldinger technique. J Cardiothorac Anesth. 2 (3), 403 (1988).
- Carbone, L. Estimating mouse and rat use in American laboratories by extrapolation from animal welfare act-regulated species. Sci Rep. 11 (1), 493 (2021).
- Su, C. S., et al. Efficacious and safe orotracheal intubation for laboratory mice using slim torqueable guidewire-based technique: Comparisons between a modified and a conventional method. BMC Anesthesiol. 16, 5 (2016).
- Clary, E. M., O'halloran, E. K., De La Fuente, S. G., Eubanks, S. Videoendoscopic endotracheal intubation of the rat. Lab Anim. 38 (2), 158-161 (2004).
- Ghali, M. G. Z. Microsurgical technique for tracheostomy in the rat. MethodsX. 5, 61-67 (2018).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved