Enhancing Efficiency and Radiolabeling Yields of Carbon-11 Radioligands for Clinical Research Using the Loop Method
In This Article
Summary
This protocol presents a detailed procedure to prepare four carbon-11 radiotracers by way of the “loop method” [11C]methylation. We describe the procedure to synthesize [11C]ER-176, with data for three additional radiotracers: [11C]MRB, [11C]mHED, and [11C]PiB. The loop method provides an efficient synthesis with increased radiochemical yields against traditional reaction vessel [11C]methylation.
Abstract
A successful positron emission tomography imaging program involving carbon-11 radiotracers demands fast, efficient, and reliable synthesis methods, requiring an on-site cyclotron and radiochemistry group, as well as clinical staff trained to operate under the unique constraints of the carbon-11 radionuclide. This study examines the merits and advantages of a captive solvent 'loop method' of radiolabeling four tracers with the carbon-11 radionuclide, producing the radioligands [11C]ER-176, [11C]MRB, [11C]mHED, and [11C]PiB.
The 'loop method' is compared against the traditional reactor-based method of carbon-11 methylation in the course of synthesizing the same radiotracers on the identical automated platform. Further, a complete overview of the clinical research preparation of the [11C]ER-176 radiotracer is presented. As demonstrated by the production of [11C]ER-176, the captive solvent 'loop method' of heterogeneous alkylation proved to be more efficient, with excellent radiochemical purity (99.6 ± 0.6%, n = 25), higher and more consistent radiochemical yield (end of synthesis (EOS) = 5.4 ± 2.2 GBq, n = 25) compared to the reactor method (EOS = 1.6 ± 0.5 GBq, n = 6), increased molar activity (loop method = 194 ± 66 GBq/µmol, n = 25; reactor method = 132 ± 78 GBq/µmol, n = 6), along with an average 5 min shorter reaction sequence.
Introduction
Among the modalities in molecular imaging, positron emission tomography (PET) is distinct in its manner of resolution of the biochemical processes associated with specific physiological targets or regions of interest1,2. The characteristic sensitivity and non-invasive nature of PET are harnessed for the in vivo visualization and quantification of disease pathophysiology, often revealing targets invisible by more anatomical imaging techniques such as computed tomography (CT)3 or magnetic resonance imaging (MRI)4. Contemporary molecular imaging sees the combination of PET with CT or MRI (PET/CT or PET/MR, respectively), leveraging PET's high-contrast resolution and quantifiable imaging parameters to provide highly accurate attenuation correction maps (PET/CT) and improved spatial resolution (PET/MR)5, overcoming some hurdles presented by variability in the higher kinetic energies of positrons from radionuclides such as gallium-68 and rubidium-826. These dual-modality imaging techniques channel the hallmark attributes of each individual modality, furnishing clinicians or researchers with a wealth of co-registered anatomical and biochemical insights into the study subject5.
The clinical applicability of this imaging technique is vast, offering visualization and measurement of molecular-level physiological processes as diverse as glucose metabolism7,8, neurotransmitter receptor binding9, myocardial perfusion10, and various neurological conditions11. Outside clinical use, the inherent attributes of PET are aligned to play an integral role in diagnostic and therapeutic drug development, allowing for the quantification of parameters such as binding potential (BP), biodistribution, volume of distribution (VT), and drug-receptor occupancy (RO%) by direct observation of the interplay of pharmacology, pharmacokinetics, and pharmacodynamics. This in turn, contributes to determinations, including whether a compound reaches a target at an effective dose (ED50) concentration, the extent of effective penetration of the blood-brain barrier, the metabolic integrity of the compound, and appropriate dose and dosing interval11.
In developing a useful probe for PET imaging, upon the identification of an appropriate biomarker and selecting an associated ligand, radiolabeling of the biomolecule with a suitable PET radionuclide yields the radiotracer probe for the PET study. Among PET radionuclides for investigating biological, pharmacological, or medical questions, carbon-11 offers a combination of synthetic versatility and favorable physical characteristics that enables its widespread use across diverse biomolecules and eligible ligands6. With a 99.8% positron emission and 20.4-minute half-life12, carbon-11 allows repeated administration to subjects within short intervals while still permitting multi-step syntheses. However, these advantages necessitate a facility with on-site cyclotron and radiochemistry capabilities5.
Such facilities require reliable, powerful, and reproducible methylation methods that enable the radiolabeling of precursor molecules, often with the electrophilic [11C]iodomethane ([11C]CH3I) or [11C]methyl triflate ([11C]CH3OTf) substrate13. A radiosynthesis module, as supplied from the manufacturer, is typically configured for a reaction vessel approach to [11C]methylation reactions14. This involves cooling the vessel for effective [11C]iodomethane or [11C]methyl triflate retention upon delivery, sealing and heating the vessel to effectuate the reaction, quenching, and then transferring the reacted contents to a high-performance liquid chromatography (HPLC) system for semipreparative purification13. While effective15, this technique presents numerous potential structural points of failure, involving vial septa, needles, and associated transfer lines.
The need for a more reliable and reproducible methylation method guided the inquiry and pursuit of a captive solvent modification to many of our carbon-11 radioligand synthesis protocols. This approach aims to address the limitations of the conventional reaction vessel method while maintaining or improving radiolabeling efficiency.
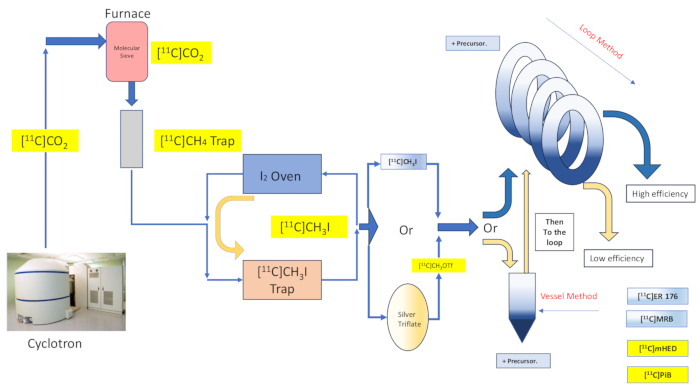
Figure 2: Design and flow of the reaction vessel synthesis and loop method. Please click here to view a larger version of this figure.
Captive solvent chemistry offers the promise of efficient trapping of, and reaction with, the radiolabeling reagent by means of spreading of the precursor solution over a large area of a supporting material or structure, and then directing the gaseous labeling reagent into contact with the coated material16,17. This enhances the extent and quality of contact between the two reactant phases16,18. Numerous implementations and variations of this technique have been documented as early as 198516,17,18,19,20, and it has found application with [11C]methyl iodide, [11C]methyl triflate, as well as Grignard reagent [11C]carbon dioxide radiolabeling reactions20. Further refinement was presented by the discussion of captive solvent "loop method" chemistry, originally described by Wilson et al., which does not require any additional supports to that already offered by the HPLC purification loop, nor heating or cooling of the reaction environment13. Captive Solvent "loop method" radiolabeling was found to impart iodomethane and methyl triflate [11C]methylation reactions with the virtues of minimal transfer loss, high radiochemical yield, high molar activity, decreased reaction time, and simplicity7,21,22,23.
Herein we describe our group's implementation of the "loop method" [11C]methylation technique originally described by Wilson13, and others thereafter14,15,18,21,22,23, (see Figure 2) by means of mechanical modification (see Figure 3) to our synthesis module (hereafter referred to as the Module). Hewing as closely to the aspirational ethos of simplicity as possible, these mechanical modifications were minimal and accessible, resulting in an overall reduction of engineering complexity, and only the addition of essential components to those already installed by the module's manufacturer as associated with a default reaction vessel radiolabeling arrangement. This is reflected in the decision to utilize the stainless steel HPLC purification loop already supplied and preinstalled by the module manufacturer, as described below, which proved compatible and effective with the syntheses examined. We discuss, in full detail, the complete and validated loop method radiolabeling protocol utilized in the clinical research production for the synthesis of the radiotracer [11C]-(R)-N-sec-butyl-4-(2-chlorophenyl)-N-methylquinazoline-2-carboxamide,[11C]ER-176 (1), using carbon-11 iodomethane. Further, we compare numerous attributes related to radiolabeling efficiency as conducted by both the reactor method and the loop method across three additional radioligands, which include (S,S)-[11C]methylreboxetine ([11C]MRB (2)),[11C]-meta-hydroxyephedrine ([11C]mHED (3)), and 2-[4-[(11C)methylamino]phenyl]-1,3-benzothiazol-6-ol ([11C]PiB (4)) developed in our facility, as determined by analysis of synthesized batches (see Table 1 and Figure 1). This comparison illustrates the clear boon to parameters such as radiochemical yield and molar activity associated with the implementation of "loop method" radiolabeling, afforded by accessible and simple module modifications of minimal cost to the radiochemistry laboratory.
Protocol
NOTE: All equipment and syntheses are performed in either a lead-shielded hotcell or minicell. CAUTION: High-energy positron-emitting particles are generated and used from the cyclotron. The area is monitored with calibrated Geiger counters, and individuals wear radiation safety-issued dosimetry rings and badges. All personnel are trained to work with high-energy radioactive materials.
All syntheses are performed on a clean and sterilized Module according to our internal standard operating procedures (SOP). The cleaning process includes the use of 1 N HCl, water, acetone, and acetonitrile for the reaction portion of the Module. Meanwhile, the formulation portion of the Module is cleaned and sterilized with water and ethanol.
1. The radiolabeling of [ 11C]ER-176 (1) by way of loop method
NOTE: For a list of the materials used in preparing [11C]ER-176 (1), please see Table of Materials.
- Replumb the Module for the loop method production (see Figure 4A-D). Attach a V8 valve to a union that directly connects into the HPLC loop. This will bypass the reaction vessel.
- Condition the methane (CH4) furnace at 350 oC for 20 min with a hydrogen gas flow of 100 mL/min prior to all productions performed on the Module. Cool to 45 oC before proceeding.
- Condition the CH4 trap at 120 oC for 20 min with a helium gas flow of 50 mL/min prior to all productions performed on the Module. Cool to 70 oC before proceeding.
- Condition the methyl iodide trap at 190 oC for 20 min with a helium gas flow of 50 mL/min prior to all productions performed on the Module. Cool to 50 oC before proceeding.
- Reagent loading for the Module:
- Using a 100 µL syringe, inject the following mixture through the adapter at position #1 of the six-port valve’s internal HPLC loop: (R)-N-sec-butyl-4-(2-chlorophenyl)-quinazoline-2-carboxamide (1.0 ± 0.05 mg) in dimethyl sulfoxide-d6 (100 ± 10 μL) and 6 N sodium hydroxide (4 μL).
NOTE: Deuterated solvents were selected for their availability in ampule form and because they are typically distilled during manufacturing, ensuring they are free from water. - Load the V4 reservoir with 3.0 mL of 0.9% sodium chloride for injection.
- Load the V5 reservoir with 1.0 mL of 200-proof ethanol.
- Load the V6 reservoir with 10 mL of sterile deionized water.
- Fill the large receiving flask with 25 mL of sterile deionized water.
- Fill the formulation flask with 6.0 mL of 0.9% sodium chloride for injection.
- Ensure the delivery line is attached to a sterile preassembled final product vial.
- Prepare the mobile phase with a 37:63 (v/v) mixture of acetonitrile and 20 mM ammonium hydroxide.
- Condition the semi-prep column with four column volumes of mobile phase.
NOTE: The column used is a C18 100 mm x 10 mm reverse-phase column.
- Using a 100 µL syringe, inject the following mixture through the adapter at position #1 of the six-port valve’s internal HPLC loop: (R)-N-sec-butyl-4-(2-chlorophenyl)-quinazoline-2-carboxamide (1.0 ± 0.05 mg) in dimethyl sulfoxide-d6 (100 ± 10 μL) and 6 N sodium hydroxide (4 μL).
- During the preparation of the automated module, bombard carbon-11 cyclotron target (1% oxygen, 99% nitrogen) on an 11 MeV cyclotron at 55 μA for 60–80 min on dual beam targets to produce [11C]carbon dioxide by the 14N(p,α) 11C nuclear reaction.
- Approximately 20 min before unloading [11C]carbon dioxide from the cyclotron to the Module, start the validated time list for [11C]ER-176 synthesis by clicking the start button. Allow the CH4 column to cool down to 45 oC before receiving radioactive [11C]carbon dioxide (see Figure 3). Allow carbosphere column trap (CH4 trap) to cool to -75 oC.
- Let the method embedded in the Module for the conversion of [11C]carbon dioxide into [11C]methyl iodide, by way of a dry chemistry process, proceed as follows:
- Convert [11C]carbon dioxide activity into [11C]methane by reacting with hydrogen gas at 350 oC over a nickel catalyst (Shimalite-Nickel). Use an ascarite trap (sodium hydroxide) to retain the unconverted [11C]carbon dioxide and formed water.
- Entrap the formed [11C]methane on a carbosphere column trap (CH4 trap) at -75 oC for further purification and concentration. To release the trapped [11C]methane, heat the carbosphere column to 80 oC.
- React the purified [11C]methane with elemental iodine at 720 oC to form [11C]methyl iodide ([11C]CH3I) by way of a helium recirculation gas pump where incident-generated hydrogen iodide is retained by another ascarite trap while unconverted [11C]methane returns into the circulation process.
- Entrap the formed [11C]CH3I at room temperature on the methyl iodide (MeI) column that has accumulated during the recirculation process.
- At the end of the circulation process, release the collected [11C]CH3I from the MeI trap by heating to 190 oC under helium flow (15 mL/min), by-passed from the MeTf column, and guided through a check-valve into the 1.5-mL stainless steel loop containing the preloaded precursor solution (step 1.5.1).
- Once the [11C]CH3I has passed through the loop for 180 s, inject the reaction mixture onto the semi-prep HPLC column for purification. Use the following HPLC conditions: mobile phase of 37:63 (v/v) acetonitrile: 20 mM ammonium hydroxide at a flow rate of 5.0 mL/min and a UV wavelength of 235 nm; retention time (tR) of [11C]ER-176: approximately 12–14 min. See Figure 5.
- Collect the fraction sample into a large receiving flask containing 25 mL of sterile deionized water. Load the diluted mixture onto a C18 light solid phase extraction (SPE) cartridge.
- Wash product (1) with an additional 10 mL of sterile deionized water.
- Elute the desired product off the C18 light SPE with the use of 200-proof ethanol (1 mL). Direct this elution into a formulation flask preloaded with 0.9% sodium chloride for injection (6 mL).
- Further rinse the C18 light SPE with an additional 3 mL of 0.9% sodium chloride for injection via V4 reservoir.
- Collect the final solution in the formulation flask (~10 mL) and pass through a 0.22-μm sterilizing filter into a preassembled sterile, apyrogenic, USP Type I 50 mL glass vial, sealed with a rubber septum and crimped with an aluminum cap.
- Using telemanipulators, remove an aliquot from the final product vial as a quality control (QC) sample. Subject this sample to numerous QC tests to ensure conformance with established specifications prior to release and administration to a patient (see Table 2).
NOTE: Apply this loop method procedure using [11C]CH3I for the radiolabeling of (2); generate and use [11C]CH3OTf to produce (3) and (4) (see Supplemental File 1).- To follow the typical quality control analysis conducted in the facility, use telemanipulators to transfer a sample from the final product vial into a TB syringe and then to the Quality Control room using a lead-shielded carrier.
- In a lead-shielded area (L-block), expel the sample into a pyrogen-free tube, and then dispense it into smaller glass vials (50–100 μL) for HPLC and gas chromatography (GC) analyses.
- Dilute a second sample with pyrogen-free water to the appropriate concentration for bacterial endotoxin analysis.
- Inoculate a sample of the final product solution (in both tryptic soy broth (TSB) and fluid thioglycolate medium (FTM) media as a sterility sample). Incubate and observe these samples for growth over 14 days.
- Apply a small aliquot to a pH strip to visually determine the acidity/basicity of the final product solution.
2. Reaction vessel method for the radiolabeling of [11C]ER-176 (1)
- Ensure the Module is thoroughly cleaned according to the internal cleaning protocol.
- Use 1 N HCl, water, acetone, and acetonitrile for cleaning the reaction portion of the Module, and water followed by ethanol to clean and sterilize the formulation portion of the Module.
- Condition the methane (CH4) furnace at 350 oC for 20 min with a hydrogen gas flow of 100 mL/min prior to all productions performed on the Module. Cool to 45 oC before proceeding.
- Condition the CH4 trap at 120 oC for 20 min with a helium gas flow of 50 mL/min prior to all productions performed on the Module. Cool to 70 oC before proceeding.
- Condition the MeI trap at 190 oC for 20 min with a helium gas flow of 50 mL/min prior to all productions. Cool to 50 oC before proceeding. Ensure the V8 tubing is connected to the needle in the vessel.
- Perform a leak test using helium gas, from the receiving valve (V24 to V16) to the internal MeI loop, and from V8 to the vessel using V23 as a source of seal/exhaust.
- Reagent loading for the Module:
- Add the following: (R)-N-sec-butyl-4-(2-chlorophenyl)-quinazoline-2-carboxamide (1.0 ± 0.05 mg) in dimethyl sulfoxide-d6 (100 ± 10 μL) and 6 N sodium hydroxide (4 μL) to reactor vessel.
- Load the V2 reservoir with 1.0 mL of 37:63 acetonitrile:20 mM ammonium hydroxide.
- Load the V4 reservoir with 3.0 mL of 0.9% sodium chloride for injection.
- Load the V5 reservoir with 1.0 mL of 200-proof ethanol.
- Load the V6 reservoir with 10 mL of sterile deionized water.
- Fill the large receiving flask with 25 mL of sterile deionized water.
- Fill the formulation flask with 6.0 mL of 0.9% sodium chloride for injection.
- Ensure the delivery line is connected to the preassembled sterile final product vial.
- Fill up a reservoir with HPLC mobile phase (37:63 (v/v) acetonitrile:20 mM ammonium hydroxide) and connect to the HPLC pump.
NOTE: The semi-prep column used is the same as above and preconditioned with the mobile phase (4 column volumes).
- During the preparation of the automated module, bombard carbon-11 cyclotron target (1% oxygen, 99% nitrogen) on an 11 MeV cyclotron at 55 μA for 60–80 min on dual beam targets to produce [11C]carbon dioxide by the 14N(p,α) 11C nuclear reaction.
- Approximately 20 min before unloading [11C]carbon dioxide from the cyclotron to the Module, start the validated time list for [11C]ER-176 synthesis by clicking the start button. Allow the CH4 column to cool down to 45 oC before receiving radioactive [11C]carbon dioxide (see Figure 3). Allow carbosphere column trap (CH4 trap) to cool to -75 oC
- Let the method embedded in the Module for the conversion of [11C]carbon dioxide into [11C]methyl iodide, by way of a dry chemistry process, proceed as follows:
- Convert [11C]carbon dioxide activity into [11C]methane by reacting with hydrogen gas at 350 oC over a nickel catalyst (Shimalite-Nickel). Retain both unconverted [11C]carbon dioxide and formed water with an ascarite trap (sodium hydroxide).
- Entrap [11C]methane on a carbosphere column at -75 oC for further purification and concentration. To release the [11C]methane, heat the carbosphere column to 80 oC.
- React the purified [11C]methane with elemental iodine at 720 oC to form [11C]methyl iodide by way of a recirculation gas pump where incident-generated hydrogen is retained by another ascarite trap while unconverted [11C]methane returns into the circulation process, called internal loop recirculation.
- Entrap the formed [11C]methyl iodide at room temperature on the MeI column that has accumulated during the recirculation process.
- At the end of the circulation process, heat the porous polymeric adsorbent trap to 190 oC under helium flow (30 mL/min) to release the collected [11C]methyl iodide and guide it through a check-valve to the 3 mL reaction vessel for the carbon-11 radiolabeling of (1).
- Once the generation and accumulation of [11C]methyl iodide have plateaued, by-pass the silver triflate column and direct the radioactive gas through V8 into the reaction vessel containing the precursor mixture. Allow the [11C]methyl iodide to bubble for 3 min, then seal the reaction vessel and heat it at 80 oC for 5 min.
- Once the labeling is complete, after 5 min, cool the reaction vessel to 30 oC and dilute with 1 mL of mobile phase from the V2 reservoir.
- Inject the mixture onto the semi-prep HPLC column for purification using the following HPLC conditions: mobile phase of 37:63 (v/v) acetonitrile: 20 mM ammonium hydroxide at a flow rate of 5.0 mL/min and a UV wavelength of 235 nm; tR of [11C]ER-176 (1): approximately 9–11 min, see Figure 6.
- Collect the fraction sample into the large receiving flask containing 25 mL of sterile deionized water. Load this diluted mixture onto a C18 light SPE cartridge.
- Wash the trapped product (1) with an additional 10 mL of sterile deionized water.
- Elute the desired product off the C18 light SPE with the use of 200 proof ethanol (1.0 mL), directed into a formulation flask preloaded with 0.9% sodium chloride for injection (6.0 mL).
- Rinse the C18 light SPE with an additional 3.0 mL of 0.9% sodium chloride for injection.
- Collect the final solution in the formulation flask (~10 mL). Pass this media through a 0.22-μm sterilizing filter into a preassembled sterile, apyrogenic, USP Type I 50 mL glass vial, sealed with a rubber septum and crimped with an aluminum cap.
- Use telemanipulators to remove an aliquot from the final product vial as a quality control (QC) sample (see step 1.15). Subject the sample to several tests in which the radiotracer must pass all acceptance criteria prior to release and administration into a patient (see Table 2).
NOTE: Apply this reactor vessel method using [11C]CH3I for the radiolabeling of (2); generate and use [11C]CH3OTf to produce (3) and (4) (see Supplemental File 1).
Representative Results
The New York University Langone Health (NYULH) Radiochemistry group provides various carbon-11, fluorine-18, and gallium-68 radiotracers used for both human research and preclinical applications. Several methods are utilized for the production of the PET radiotracers. Our team employs the loop method for the synthesis of (1), (2), (3), and (4) (see Figure 1 and Figure 8). After production is complete, an aliquot is removed from the sterile final product vial. This sample is used for both the inoculation of the final product solution (in both tryptic soy broth (TSB) and fluid thioglycolate medium (FTM) media as a sterility sample) as well as a representative sample of the bulk solution for QC tests. Each radiotracer is subjected to QC tests before the product is released for administration (see Table 2).
Quality control testing includes the visualization of product appearance, verification of filter integrity, determination of radionuclidic identity, pH, radiochemical identity (radio-HPLC), radiochemical purity (radio-HPLC), chemical purity (HPLC), molar activity, strength, residual solvent, endotoxins, and sterility (see Table 2). The following results were obtained from the clinical production of each above-mentioned radiotracer (see Table 1).
For a representative analytical HPLC chromatogram, see Figure 7 and Supplemental File 1. Every radiotracer is required to pass all quality control specifications (see Table 2) before they can be released and administered to a subject.
Refer to the Supplemental File 1 for amounts of precursor and reagents as well as the analytical HPLC chromatograms of [11C]MRB (2) (Supplemental Figure 1), [11C]mHED (3) (Supplemental Figure 2), and [11C]PiB (4) (Supplemental Figure 3).
| Compound | Parameters | Loop Method (avg ± std) | Reactor Method (avg ± std) |
| [11C]ER-176 | number of productions | 25 | 6 |
| Start of Synthesis | 86 ± 5.0 GBq | 52 ± 25.7 GBq | |
| End of Synthesis | 5.4 ± 2.2 GBq | 1.6 ± 0.5 GBq | |
| Radiochemical Purity | 99.6 ± 0.6% | 99.9 ± 0.1% | |
| ER-176 concentration | 1.1 ± 0.5 μg/mL | 0.63 ± 0.37 μg/mL | |
| molar activity | 194 ± 66 GBq/μmol | 132 ± 78 GBq/μmol | |
| Total Synthesis Time | 36 ± 3 min | 44 ± 6 min | |
| [11C]MRB | number of productions | 70 | 6 |
| Start of Synthesis | 84 ± 5.4 GBq | 39 ± 11.9 GBq | |
| End of Synthesis | 3.0 ± 1.2 GBq | 1.9 ± 0.7 GBq | |
| Radiochemical Purity | 99.5 ± 0.5% | 99.7 ± 0.8% | |
| MRB concentration | 0.52 ± 0.24 μg/mL | 0.68 ± 0.41 μg/mL | |
| molar activity | 190 ± 50 GBq/μmol | 99 ± 55 GBq/μmol | |
| Total Synthesis Time | 35 ± 3 min | 42 ± 3 min | |
| [11C]mHED | number of productions | 5 | 11 |
| Start of Synthesis | 69 ± 10.5 GBq | 82 ± 4.3 GBq | |
| End of Synthesis | 5.5 ± 1.3 GBq | 3.3 ± 1.0 GBq | |
| Radiochemical Purity | 98.2 ± 1.3% | 99.1 ± 0.7% | |
| mHED concentration | 0.40 ± 0.10 μg/mL | 0.52 ± 0.37 μg/mL | |
| molar activity | 301 ± 48 GBq/μmol | 155 ± 77 GBq/μmol | |
| Total Synthesis Time | 27 ± 4 min | 32 ± 2 min | |
| [11C]PiB | number of productions | 51 | 10 |
| Start of Synthesis | 86 ± 5.4 GBq | 57 ± 17.2 GBq | |
| End of Synthesis | 3.2 ± 0.8 GBq | 1.4 ± 0.2 GBq | |
| Radiochemical Purity | 97.0 ± 1.5% | 99.1 ± 1.4% | |
| PiB concentration | 0.22 ± 0.51 μg/mL | 0.30 ± 0.24 μg/mL | |
| molar activity | 201 ± 68 GBq/μmol | 207 ± 124 GBq/μmol | |
| Total Synthesis Time | 35 ± 2 min | 36 ± 5 min |
Table 1: Results from the production of [11C]ER-176 (1), [11C]MRB (2), [11C] m HED (3), and[11C]PiB (4) by way of loop method or reaction vessel method. All values are reported at end-of-synthesis. Abbreviations: [11C]ER-176 = [11C]-(R)-N-sec-butyl-4-(2-chlorophenyl)-N-methylquinazoline-2-carboxamide; [11C]MRB = (S,S)-[11C]methylreboxetine; [11C]mHED = [11C]-meta-hydroxyephedrine; [11C]PiB = 2-[4-[(11C)methylamino]phenyl]-1,3-benzothiazol-6-ol.
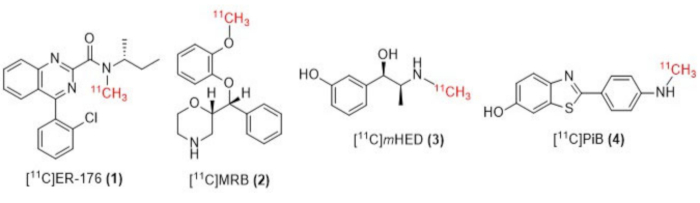
Figure 1: Structures of [11C]ER-176 (1), [11C]MRB (2), [11C] m HED (3), and [11C]PiB (4). Abbreviations: [11C]ER-176 = [11C]-(R)-N-sec-butyl-4-(2-chlorophenyl)-N-methylquinazoline-2-carboxamide; [11C]MRB = (S,S)-[11C]methylreboxetine; [11C]mHED = [11C]-meta-hydroxyephedrine; [11C]PiB = 2-[4-[(11C)methylamino]phenyl]-1,3-benzothiazol-6-ol. Please click here to view a larger version of this figure.
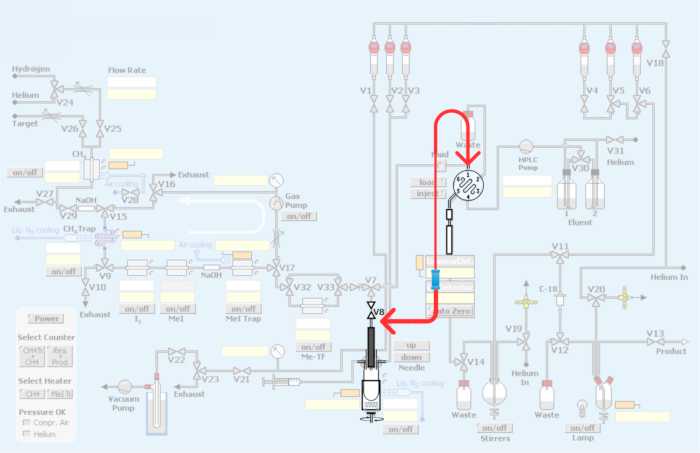
Figure 3: Modifications made to the automated Module. In red represents the replumbing of the synthesis module to incorporate the loop for the production of PET radiotracers by way of carbon-11 methylation. Please click here to view a larger version of this figure.
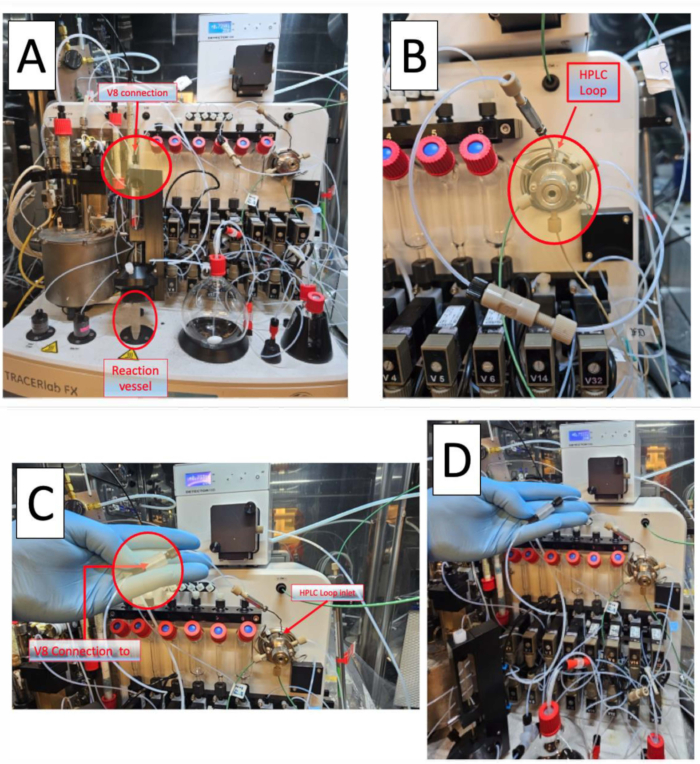
Figure 4: Module plumbing and re-plumbing to incorporate the loop method for carbon-11 methylation of PET radiotracers. (A) HPLC injection loop with union connectors. (B) Reaction vessel method. The red arrow showing the V8 connection. (C) Loop method re-plumbing, by-passing the reactor vessel. Note that the V8 connection to the HPLC inlet bypasses the reaction vessel, allowing [11C]iodomethane or [11C]methyl triflate direct access to the HPLC loop. (D) Placement of the union in regards to reactor vessel. Note the connection to the stainless steel HPLC loop. Please click here to view a larger version of this figure.
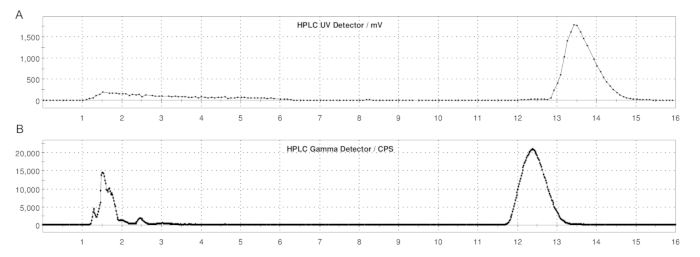
Figure 5: Semi-prep HPLC profiles of ER-176 and [11C]ER-176 with loop method production. (A) Semi-prep HPLC profile for ER-176 (1) by way of loop method production at UV = 235 nm; tR = 13.2 min. (B) Semi-prep radio-HPLC profile for [11C]ER-176 (1); tR = 12.4 min. Conditions: mobile phase of 37:63 (v/v) acetonitrile: 20 mM ammonium hydroxide at a flow rate of 5.0 mL/min. Abbreviation: [11C]ER-176 = [11C]-(R)-N-sec-butyl-4-(2-chlorophenyl)-N-methylquinazoline-2-carboxamide. Please click here to view a larger version of this figure.
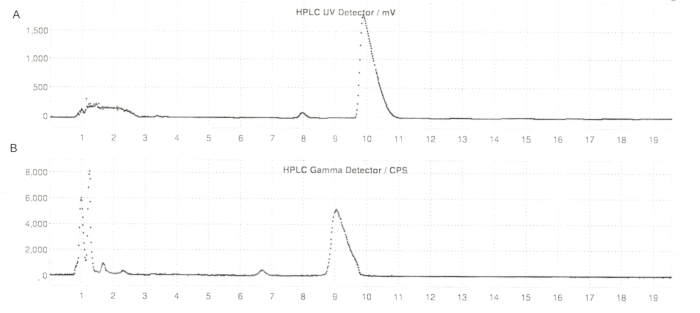
Figure 6: Semi-prep HPLC profiles of ER-176 and [11C]ER-176 with reactor method production. (A) Semi-prep HPLC profile for ER-176 (1) by way of reactor method production at UV = 235 nm; tR = 9.8 min. (B) Semi-prep radio-HPLC profile for [11C]ER-176 (1); tR = 9.2 min. Conditions: mobile phase of 37:63 (v/v) acetonitrile: 20 mM ammonium hydroxide at a flow rate of 5.5 mL/min. Abbreviations: [11C]ER-176 = [11C]-(R)-N-sec-butyl-4-(2-chlorophenyl)-N-methylquinazoline-2-carboxamide; tR = retention time. Please click here to view a larger version of this figure.
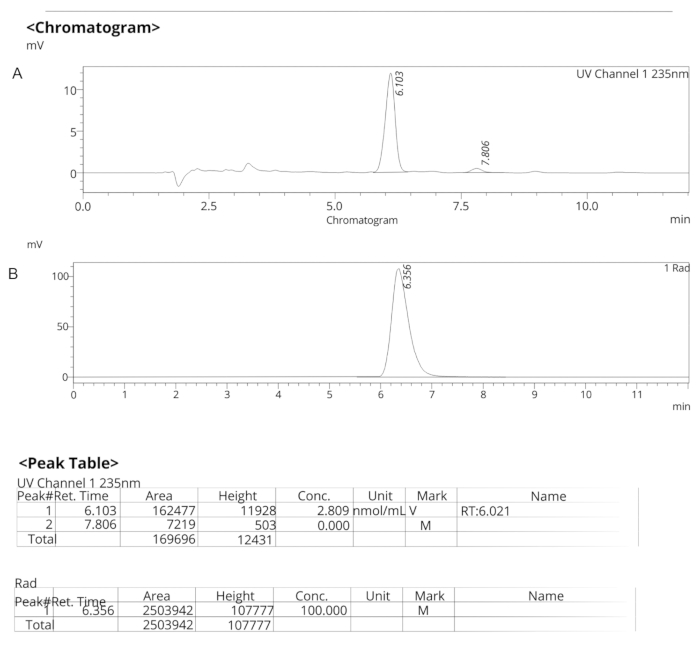
Figure 7: Analytical HPLC profiles for ER-176. (A) Analytical HPLC profile of UV spectra for ER-176 (1) at 235 nm; tR = 6.10 min. (B) Analytical HPLC profile of radiotracer for [11C]ER-176 (1); tR = 6.36 min. Conditions: 10 µm C18 (2) 100  LC Column 250 x 4.6 mm; methanol/water 74/26 with a flow rate of 1.5 mL/min. Abbreviation: [11C]ER-176 = [11C]-(R)-N-sec-butyl-4-(2-chlorophenyl)-N-methylquinazoline-2-carboxamide; tR = retention time Please click here to view a larger version of this figure.
LC Column 250 x 4.6 mm; methanol/water 74/26 with a flow rate of 1.5 mL/min. Abbreviation: [11C]ER-176 = [11C]-(R)-N-sec-butyl-4-(2-chlorophenyl)-N-methylquinazoline-2-carboxamide; tR = retention time Please click here to view a larger version of this figure.
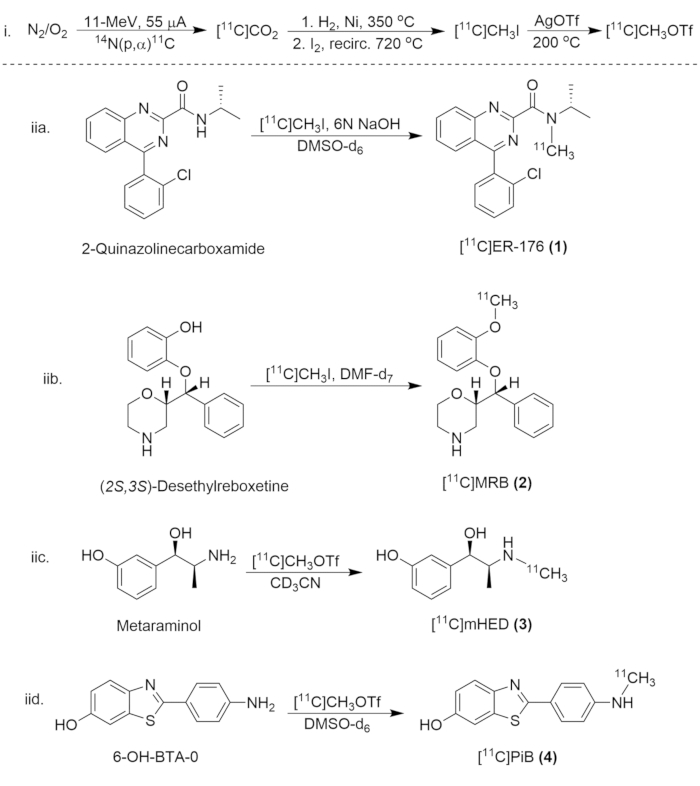
Figure 8: Synthetic Scheme for Synthesis of Carbon-11 Compounds. (i) The formation of [11C]CH3I and [11C]CH3OTf, and (iia.) the radiolabeling of [11C]ER-176 (1), (iib.) [11C]MRB (2), (iic.)[11C]mHED (3), and (iid.) [11C]PiB (4). Please click here to view a larger version of this figure.
| Test/Parameters | Specifications |
| Appearance (visual inspection) | Clear, colorless solution free from particulate matter |
| Membrane filter integrity | ≥ 50 psi |
| pH | 4.5 - 8.0 |
| Radionuclidic identity (half-life) | 19.3 - 21.3 min |
| Radiochemical identity (radio-HPLC) | 0.90 ≤ tR Prod / t R Std ≤ 1.10 |
| Radiochemical purity (radio-HPLC) | ≥ 95.0%† |
| Drug concentration (HPLC) | see notes* |
| Total chemical impurities (HPLC) | see notes** |
| Molar activity (@ EOS) | > 9.25 GBq/μmol |
| Residual acetonitrile (GC) | ≤ 410 ppm |
| Residual acetone (GC) | ≤ 5,000 ppm |
| Residual dimethyl sulfoxide (GC) | ≤ 5,000 ppm |
| Residual ethanol (GC) | ≤ 10% (v/v) |
| Residual methanol (GC) | ≤ 3,000 ppm |
| Residual N,N-dimethylformamide (GC) | ≤ 880 ppm |
| Limulus Amoebocyte Lysate (LAL) | ≤ 17.5 EU/mL |
| Sterility (initiate within 30 h) | Sterile (14 days) |
| Preparation records | Complete and accurate |
| Labels | Complete, accurate, reconciled |
Table 2: Quality control criteria for the approval or rejection of carbon-11 radiotracers. † Radiochemical purity ≥ 90.0% for [11C]mHED; * Drug Concentration: [11C]ER-176 ≤ 10 µg/dose; [11C]MRB ≤ 10 µg/dose; [11C]mHED ≤ 50 µg/dose; [11C]PiB ≤ 13.4 µg/dose; ** Total chemical impurities: [11C]ER-176 ≤ 1.0 µg/dose; [11C]MRB ≤ 1.0 µg/dose; [11C]mHED ≤ 5.0 µg/dose; [11C]PiB ≤ 1.34 µg/dose. Abbreviations: [11C]ER-176 = [11C]-(R)-N-sec-butyl-4-(2-chlorophenyl)-N-methylquinazoline-2-carboxamide; [11C]MRB = (S,S)-[11C]methylreboxetine; [11C]mHED = [11C]-meta-hydroxyephedrine; [11C]PiB = 2-[4-[(11C)methylamino]phenyl]-1,3-benzothiazol-6-ol; EOS = end of synthesis; GC = gas chromatography; LAL = Limulus Amebocyte Lysate.
Supplemental File 1: Chemicals and materials, reagents used for loop or reactor vessel method, Quality Control testing, analytical HPLC chromatograms. Please click here to download this file.
Discussion
Traditional radiolabeling of terminal heteroatoms with carbon-11 iodomethane or methyl triflate involves bubbling the radioactive electrophilic gas into a reaction vessel, trapping it, and allowing the solution to react for extended periods of time18. The conventional bubbling of the heterogeneous reaction can be sluggish and may require heating to accelerate the reaction rate. Before purification, cooling the reaction media to room temperature may be necessary, followed by transferring to an HPLC loop with the use of solvent (e.g., mobile phase) for purification of the desired radiotracer. These steps are time-consuming, and fleeting radiolabeled products may be lost during the transfer of such materials.
At our facility, we have demonstrated the justification for using the loop method, where precursor solution is coated on an HPLC stainless steel loop, and the radiolabeling of the compound takes place inside the loop at room temperature. The loop is tied in-line with the delivery of the radioactive [11C]CH3I or [11C]CH3OTf and connected to the injection port of an HPLC system. No heating is required for the radiolabeling to occur, and for all cases shown in this manuscript, the reaction takes place in under 3 min.
The flow rate and size of the stainless steel HPLC loop appear to be critical for this process to work efficiently. Testing began by varying the flow rate of the carrier gas, helium, from 8.0 mL/min to 15.0 mL/min for the radioactive gas to be delivered to the loop. Buckley studied the importance of applying the correct flow rate as well as using the appropriate solvent and loop material15. For our system, the flow rate of 15 mL/min for either radioactive electrophilic species of [11C]CH3I or [11C]CH3OTf performs well for the radiolabeling of all four radiotracers discussed in this manuscript. The loop used for all syntheses is a stainless steel HPLC injection loop at 1.5 mL possessing an OD of 1/16 inch and an ID of 1 mm.
Comparing the two methods (reaction vessel versus loop method), in our hands, the loop method demonstrated increased radiolabeling efficiency along with a substantial increase in molar activity at end-of-synthesis (EOS) for the production of four human research-approved radiotracers. As an example, the radiotracer [11C]mHED (3) had a 1.6-fold increase in the isolated final product activity along with achieving double the molar activity at EOS. This trend of increased overall activity is observed across all four radiotracers (see Table 1). Additional favorable outcomes when using the loop method include a reduced setup time by 5 min and no need to clean the reaction vessel, saving the operator's time and use of solvents for a cleaning protocol.
Some drawbacks to this methodology include the limits on which radiotracers can be effectively labeled using the loop method. If heat is necessary for the radiolabeling, it is difficult to modify this system to allow for heating inside the HPLC loop. This system requires modifications to the plumbing; this feature of not having it ready 'out of the box' may deter other users from performing such alterations to their automated platform14. Since supplemental plumbing and unions are required for this method (see Figure 4A-D), the potential for additional sites for radioactive releases increases when labeling under these conditions. It is prudent to perform a leak check prior to every run on the Module.
Our team has implemented the loop method used in the Investigational New Drug Application (IND) and Radioactive Drug Research Committee (RDRC)-approved production of four carbon-11 radiotracers. In our hands, this method proved to be a more efficient and higher-yielding process than the traditional reaction vessel method. Additional plumbing and adjustments to the flow rate of the carrier helium gas need to be considered when applying this method to most automated Modules. Finally, this method has limitations and is not suitable for certain carbon-11 radiotracers, such as [11C]UCB-J, which requires activation of the palladium (II) intermediate and heating24 of the reaction mixture.
Disclosures
The authors declare they have no relevant or financial interests related to this research to disclose.
Acknowledgements
We would like to acknowledge former NYULH Radiochemistry lab members, Raul Jackson and Grace Yoon, for their work on initial efforts for the carbon-11 methylation using a loop method.
Materials
| Name | Company | Catalog Number | Comments |
| 2-(4'-Aminophenyl)-6-hydroxybenzothiazole | ABX | 5101 | Precursor for PiB synthesis (6-OH-BTA-0) |
| (2S,3S)-Desethylreboxetine | ABX | 4407 | Precursor for MRB synthesis |
| Acetic acid | Sigma Aldrich | 695092 | Reagent used for the synthesis of [11C]PiB |
| Acetone-d6 | Sigma Aldrich | 444863 | Solvent used for the synthesis of [11C]PiB |
| Acetonitrile, HPLC-grade | Sigma Aldrich | 34998 | Various concentrations used in the mobile phase for radiotracer productions |
| Acetonitrile-d3 | Sigma Aldrich | 151807 | Solvent used for the synthesis of [11C]mHED |
| Ammonium formate | Sigma Aldrich | 798568 | Reagent used for the synthesis of [11C]MRB |
| Ammonium hydroxide | Ricca Chemical | 642-16 | Reagent used for the synthesis of [11C]ER-176 |
| Analytical balance | Mettler Toledo | M-XS104 | Balance used to weigh out materials for productions |
| Ascarite II | Thermo Fisher | CN-C049U90 | Used in drying columns for FxC Pro Module |
| Biosafety cabinet | Comecer | M-BH4 | Used for FPV assembly |
| (R)-N-sec-Butyl-4-(2-chlorophenyl)-quinazoline-2-carboxamide | ABX | 1665.0001 | ER-176 precursor |
| C18 Light Sep-Pak cartridge | WATERS | WAT023501 | Solid phase extraction cartridge used in the synthesis of carbon-11 radiotracers |
| Carboxen, 60 – 80 mesh | Supelco | CN-10478-U | Used in the FxC Pro Module |
| Compressed – NOS (99% nitrogen/1% oxygen) | Airgas | CN-X02NI99C3003091 | Target gas used in cyclotron bombardment |
| Dispensing hot cell (DHC) | Comecer | M-MIP1-1390 | Dispensing radiotracers |
| Dose calibrator | Capintec | M-CRC-55t | Measuring activity of radiotracers |
| Endosafe nexgen-PTS | Charles River | PTS150 | Endosafe PTS, portable test sytem |
| Endotoxin PTS - Limulus Amebocyte Lysate (LAL) Test Cartridge | Charles River | PTS20F | LAL cartridges used to test endotin levels on radiotracers |
| Ethanol, 200 proof, HPLC-grade | Sigma Aldrich | 459828 | Used for final product and mobile phase of ER, MRB, mHED and PiB |
| Gas Chromatogram 2030 | Shimadzu | M-GC-2030 | Measure excipients from radiotracer productions |
| Graphpac | Supelco, Millipore Sigma | 10258 | Used in FxC Pro Module for the synthesis of [11C]ER-176, [11C]MRB, [11C]mHED and [11C]PiB |
| Helium, research grade | Airgas | CN-HER-300 | Helium tank, 99.9999% (research grade tank) |
| Shimadzu LC-20 Series | Shimadzu | Various | Analytical HPLC system |
| Hydrogen, ultra high purity grade | Airgas | CN-HYUHP-300 | Hydrogen tank used for the FxC Pro Module |
| Iodine | Thermo Fisher | I35 | Used in FxC Pro Module, conversion of [11C]CH4 to [11C]CH3I |
| Luna 10 mm C18 (2) 100 Å 250 x 10 mm column | Phenomenex | 00G-4253-N0 | Semi-prep column for MRB synthesis |
| Luna 5 mm C18 (2) 100 Å 250 x 10 mm column | Phenomenex | 00G-4252-N0 | Semi-prep column for PiB synthesis |
| Macherey-Nagel MN VP 250/10 Nucleosil 100-5 C18 Nautilus column | Macherey-Nagel | 715412.1 | Semi-prep column for mHED synthesis |
| Metaraminol (free base) | ABX | 3380.0001 | Precursor used in the production of [11C]mHED |
| Milli-Q Direct 8 DI system | Millipore | M-ZROQ00800 | De-ionized water system |
| N,N-Dimethylformamide-d7 | Sigma Aldrich | 189979 | Solvent used for the synthesis of [11C]MRB |
| Needle, 18 G x 1 | Becton Dickinson | BD 305195 | Needles used at various stages of production setup |
| Needle, 20 G x 1-1/2” | Becton Dickinson | BD 305176 | Needles used at various stages of production setup |
| Needle, 22 G x 4”, Spinal | Air-Tite Products | N224 | Needles used at various stages of production setup |
| Onyx Monolithic C18 100 x 10 mm column | Phenomenex | CH0-7878 | Semi-prep column for ER-176 synthesis |
| Phosphorus Pentoxide with Sicapent indicator | Sigma Aldrich | 79610 | Used in the synthesis Modules for carbon-11 radiotracer productions |
| Porapak N | Waters | WAT027047 | Material used to pack MeI column in FxC Pro Module |
| Pressure gauge | Omega | M-DPG1100B-100G | Used for filter integrity test |
| Shimalite-Ni | Shimadzu Corporation | 221-66062 | Material packed in CH4 oven of FxCPro Module for conversion from [11C]CO2 to [11C]CH4 |
| Siemens Eclipse 11-MeV cyclotron | Siemens | RDS 111 | Particle accelerator used to generate [11C]CO2 gas for carbon-11 productions |
| Silver Triflate | Sigma Aldrich | 483346 | Material packed in triflate column of the FxC Pro Module for the production of [11C]mHED and [11C]PiB |
| Sodium chloride injection (saline, 0.9%), 50 mL | Hospira | 0409-4888-50 | Saline used in the formulation process of radiotracers |
| Sodium chloride injection (saline, 0.9% ), 500 mL | Braun | L8001 | Reagent used in the synthesis of [11C]mHED, and mobile phase. |
| Sodium hydroxide | Sigma Aldrich | 221465 | Reagent used in the synthesis of [11C]ER-176 and other components at different concentration. |
| Sodium iodide detector | Eckert & Ziegler | PMT/Na-BFC3200 | Gamma detector used in-line with HPLC unit |
| Sterile vial, 50 mL | ALK Allergy | SEV50 | 50 mL sterile vials used as the |
| Stainless steel loop | GE | 980314/IEG-005118 | HPLC injection loop at 1.5 mL possessing an OD 1/16”, ID: 1 mm. |
| Syringe, 1 mL | Braun | NJ-9166017-02 | Syringe used at various stages of production set-up |
| Syringe, 10 mL | Henke Sass Wolf | 4100-X00V0 | Syringe used at various stages of production set-up |
| Syringe, 20 mL | Henke Sass Wolf | 4200-X00V0 | Syringe used at various stages of production set-up |
| Syringe, 5 mL | Becton Dickinson | 309632 | Syringe used at various stages of production set-up |
| TracerLab FX2C Module | GE | M-P5360QB | Automated module of production of carbon-11 radiotracers |
| Triethylamine | Sigma Aldrich | 471283 | Reagent used for the synthesis of [11C]PiB |
| Tuberculin Syringe, with 21 G x 1 Needle, Single-Use, Sterile (1 mL) | Becton Dickinson | BD 309624 | Syringe used in pre-assembled final product vials |
References
- Bailey, D. L., Townsend, D. W., Valk, P. E., Maisey, M. N. . Positron emission tomography: Basic sciences. , 223-231 (2005).
- Berger, A. How does it work? Positron emission tomography. BMJ. 326 (7404), 1449 (2003).
- Seeram, E. Computed tomography: A technical review. Radiol Technol. 89 (3), 279CT-302CT (2018).
- Grover, V. P., et al. Magnetic resonance imaging: Principles and techniques: Lessons for clinicians. J Clin Exp Hepatol. 5 (3), 246-255 (2015).
- Rowe, S. P. P., Martin, G. Molecular imaging in oncology: Current impact and future directions. CA Cancer J Clin. 72 (4), 333-352 (2022).
- Ziessman, H. A., O'Malley, J. P., Thrall, J. H. . Nuclear medicine: The requisites. , (2014).
- Cochran, B. J., et al. Determining glucose metabolism kinetics using 18F-FDG micro-PET/CT. J Vis Exp. (123), e55184 (2017).
- Pantel, A. R., Bae, S. -. W., Li, E. J., O′Brien, S. R., Manning, H. C. PET imaging of metabolism, perfusion, and hypoxia: FDG and beyond. Cancer J. (Philadelphia, PA, U. S.). 30 (3), 159-169 (2024).
- Hansen, J. Y., et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat Neurosci. 25 (11), 1569-1581 (2022).
- Valenta, I. S., Thomas, H. PET-determined myocardial perfusion and flow in coronary artery disease characterization. J Med Imaging Radiat Sci. 55 (2S), S44-S50 (2024).
- Nerella, S. G., Singh, P., Sanam, T., Digwal, C. S. PET Molecular Imaging in Drug Development: The Imaging and Chemistry Perspective. Front Med (Lausanne). 9, 812270 (2022).
- Dolle, F. Carbon-11 and fluorine-18 chemistry devoted to molecular probes for imaging the brain with positron emission tomography. J Label Compd Radiopharm. 56 (3-4), 65-67 (2013).
- Wilson, A. A., Garcia, A., Jin, L., Houle, S. Radiotracer synthesis from [11C]-iodomethane: A remarkably simple captive solvent method. Nucl Med Biol. 27 (6), 529-532 (2000).
- Bruton, L., Scott, P. J., Kilbourn, M. Automated synthesis modules for PET radiochemistry. Handb Radiopharm. (2nd Ed.). , 437-456 (2021).
- Studenov, A. R., Jivan, S., Adam, M. J., Ruth, T. J., Buckley, K. R. Studies of the mechanism of the in-loop synthesis of radiopharmaceuticals. Appl Radiat Isot. 61 (6), 1195-1201 (2004).
- Jewett, D. M., Ehrenkaufer, R. L., Ram, S. A captive solvent method for rapid radiosynthesis: application to the synthesis of [1-11C]palmitic acid. Int J Appl Radiat Isot. 36 (8), 672-674 (1985).
- Watkins, G. L., Jewett, D. M., Mulholland, G. K., Kilbourn, M. R., Toorongian, S. A. A captive solvent method for rapid N-[11C]methylation of secondary amides: application to the benzodiazepine, 4'-chlorodiazepam (RO5-4864). Appl Radiat Isot. 39 (5), 441-444 (1988).
- Iwata, R., et al. A simple loop method for the automated preparation of [11C]raclopride from [11C]methyl triflate. Appl Radiat Isot. 55 (1), 17-22 (2001).
- Iwata, R., et al. On-line [11C]methylation using [11C]methyl iodide for the automated preparation of 11C-radiopharmaceuticals. Appl Radiat Isot. 43 (9), 1083-1088 (1992).
- Mccarron, J. A., Turton, D. R., Pike, V. W., Poole, K. G. Remotely-controlled production of the 5-HT1A receptor radioligand [carbonyl-11C]WAY-100635. J Labelled Compd Radiopharm. 38 (10), 941-953 (1996).
- Shao, X., et al. Highlighting the versatility of the Tracerlab synthesis modules. Part 2: fully automated production of [11C]-labeled radiopharmaceuticals using a Tracerlab FXC-Pro. J Labelled Compd Radiopharm. 54 (14), 819-838 (2011).
- Dahl, K., Halldin, C., Schou, M. New methodologies for the preparation of carbon-11 labeled radiopharmaceuticals. Clin Transl Imaging. 5 (3), 275-289 (2017).
- Shao, X., Schnau, P. L., Fawaz, M. V., Scott, P. J. Enhanced radiosyntheses of [C]raclopride and [C]DASB using ethanolic loop chemistry. Nucl Med Biol. 40 (1), 109-116 (2013).
- Milicevic Sephton, S., et al. Automated radiosynthesis of [11C]UCB-J for imaging synaptic density by positron emission tomography. J Label Compd Radiopharm. 63 (3), 151-158 (2020).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved