A subscription to JoVE is required to view this content. Sign in or start your free trial.
The CApillary FEeder (CAFE) Assay: A Method to Track Food Consumption and Preference in Drosophila
Overview
This video describes the CApillary FEeder, or CAFE, assay, a behavioral method that measures food intake and preference of unrestrained fruit flies. The featured protocol shows how to perform the assay with minimal evaporation from the liquid-filled capillaries used to deliver the food and track its intake.
Protocol
This protocol is an excerpt from Diegelmann et al., The CApillary FEeder Assay Measures Food Intake in Drosophila melanogaster, J. Vis. Exp. (2017).
1. Assembly and Performing the CApillary FEeder Assay
- If fasting is not needed, transfer the experimental flies to the assay by tapping or by blow-pipe. Make sure to include three control vials without flies to quantify evaporation.
- Carefully remove a pipette tip (2 - 20 µL volume) that is closing one of the outer openings, and insert a filled glass capillary, bottom-end first. Secure the capillary by placing the pipette tip back next to the capillary. If several food solutions are being tested, repeat this procedure accordingly.
- Place the capillary ends inside all vials at the same level to avoid bias that could occur if the food sources were located at different heights (3 - 4 cm from the lid); keep a distance to the filter paper to prevent the capillary from leaking by accidently touching the filter paper or different viscosities of food sources.
- Label the upper end of the colored liquid using a marker pen (markbeginning). To ensure the different capillaries can be identified, label them individually using a color or stripe code.
- Place multiple prepared CAFE assays inside a plastic box with gridded inlay and transfer the box (Figure 2A) to a secure position under laboratory conditions or in a temperature-, light- and humidity-controlled climate chamber (parameters: 25 °C, 60% relative humidity, 12 h/12 h light-dark cycle) for the experimental period (e.g. 3 h or days).
- As bottom filter paper dries out if the assay is performed over several days, apply fresh water every 24 h via the sponge bung (100 µL) to keep humidity constant inside the assay. Use four separate vials (8 cm height, 3.3 cm diameter) filled with 30 mL ddH2O as humidity devices and place them next to the CAFE assays in the plastic box. Use a cover for the plastic box to create humidity controlled environment during the experiment (Figure 2A).
NOTE: Broader variability occurs under laboratory conditions; however, it is feasible to perform the CAFE assay at room temperature (e.g., in a classroom). The use of a humidification device (filter paper, with or without a wet sponge bung, filled water vials and cover for the plastic box) is highly encouraged to decrease evaporation (Figure 2B). - Replace the capillaries with freshly filled ones for long term experiments every 24 h. Make note of dead flies before each 24 h interval and use the number of live flies to calculate consumption per fly for the following period. Discard the old capillaries after measuring the decline of the meniscus (see 2.1).
NOTE: During a 3 h experiment we hardly see any dead flies. During a 4 days study we usually find 1 - 3 dead flies. - At the end of the assay or before replacing the capillary, mark the lower meniscus of the capillary (markend) with a marker pen while the CAFE assay is still in the upright position. Discard the data if markend is not below the initial mark (markbeginning). Do not remove the lid, as this might change the meniscus.
- Carefully remove the capillaries from the assay and store them for data collection. Check if the liquid inside the capillary reached the lower end if not discard the data, as food was not accessible to the flies. Collect all capillaries per vial as a group. Insert uncut pipette tips into all openings to prevent flies from escaping. Dismantle the setup and wash the vials, lids and sponge bungs in a soap bath and dry overnight at room temperature for further use.
NOTE: Flies can be further analyzed after the assay. Confirm food uptake by eye or under a dissection microscope. - Repeat experiments with the same genotypes on at least three different days.
2. Data Collection and Analysis
- Measure the distance between markbeginning and markend on the capillary using a caliper or a ruler. To transfer data directly to a spreadsheet, use a USB (Universal Serial Bus) connected digital caliper (Figure 1E). Discard the capillaries after the measurement.
- Account for capillary size to calculate food uptake or evaporation. For example, consider a capillary that is 73 mm long and contains 5 µL of food solution. A 14.6 mm decrease in the meniscus reflects the uptake of 1 µL solution. Calculate food uptake using the following formula:
Food uptake (µL) = measured distance (mm)/ 14.6 mm - To exclude the effect of evaporation on food intake, calculate mean evaporation in the three (at minimum) control vials without flies. Subtract this mean value from the value obtained for food consumption by the flies.
- Use the following formula to determine total consumption per fly:
Food consumption (µL) = (Food uptake [µL] - Evaporative loss [µL])/total number of flies in the vial. For long-term experiments use the number of flies alive before the start of the 24 h interval. - To account for differences in body size, such as between male and female flies, normalize food consumption to body weight (µL food/mg fly).
- Use statistical software for data analysis. For normally distributed data, use student's T-tests to determine differences between two fly groups, and use ANOVA (analysis of variance) with post hoc Tukey Cramer tests for more than two groups. In a choice situation, analyze differences from random choice using a nonparametric one-sample sign test.
النتائج
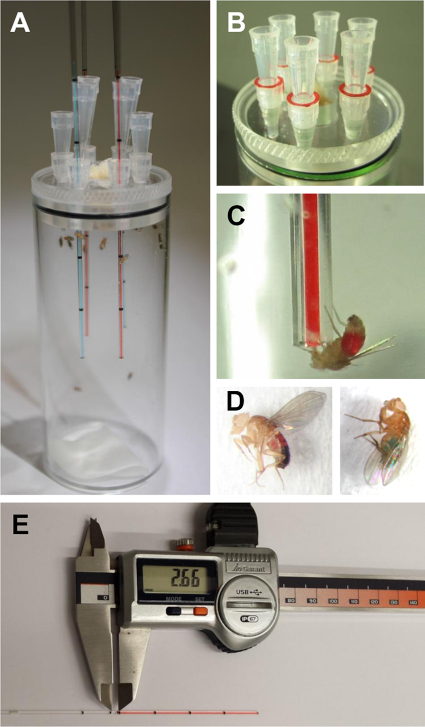
Figure 1: The Drosophila melanogaster CApillary FEeder Assay. A) The feeding assay with flies. Moistened filter paper provides water at the bottom of the vial. Four capillaries are provided during the experiment (red- and blue-colored food in opposite capillaries). Note that the capillaries are secured in position by a second pipette tip, and unused positions are closed using pipette tips. A...
Materials
| Name | Company | Catalog Number | Comments |
| Vials (breeding) | Greiner Bio-One | 960177 | www.greinerbioone.com |
| Vials (CAFE assay) | Greiner Bio-One | 217101 | www.greinerbioone.com |
| Lid-CAFE assay | Workshop | – | – |
| Plastic box, low wall | Plastime | 353 | www.plastime.it |
| Cover for the plastic box | Workshop | – | – |
| Capillaries | BLAUBRAND | REF 7087 07 | www.brand.de |
| Pipette tips | Greiner Bio-One | 771290 | www.greinerbioone.com |
| Filter paper circles | Whatman | 10 311 804 | www.sigmaaldrich.com |
| D(+)-Sucrose | AppliChem | 57-50-1 | www.applichem.com |
| Ethanol absolute | VWR Chemicals | 20,821,330 | www.vwr.com |
| Food color (red, E124) | Backfun | 10027 | www.backfun.de |
| Food color (blue, E133) | Backfun | 10030 | www.backfun.de |
| Soap solution (CVK 8) | CVH | 103220 | www.cvh.de |
| Digital caliper | GARANT | 412,616 | www.hoffmann-group.com |
| Vials (breeding) | Height 9.8 cm, diameter 4.8 cm | ||
| Vials (CAFE assay) | Height 8 cm, diameter 3.3 cm | ||
| Lid-CAFE assay | Produced in university workshop, technical drawing supplied Please click here to download this file. | ||
| Plastic box, low wall | A plastic grid inlay was custom-made for 8 x 10 vial positions | ||
| Cover for the plastic box | Dimensions (37 x 29 x 18 cm) | ||
| Capillaries | DIN ISO 7550 norm, IVD-guideline 98/79 EG, ends polished | ||
| Pipette tips | Pipettes for the outer circle are cut according to the lid | ||
| Filter paper circles | 45 mm diameter works nicely if folded for the vials used | ||
| D(+)-Sucrose | Not harmful | ||
| Ethanol absolute | Highly flammable liquid and vapor | ||
| Food color (red, E124) | Not stated | ||
| Food color (blue, E133) | Not stated | ||
| Soap solution (CVK 8) | Odor neutral soap | ||
| Digital caliper | |||
| Standard fly food | (for 20 L) | ||
| Agar | 160 g | ||
| Brewer's Yeast | 299.33 g | ||
| Cornmeal | 1,200 g | ||
| Molasses | 1.6 L | ||
| Propionic acid | 57.3 mL | ||
| Nipagin 30% | 160 mL |
This article has been published
Video Coming Soon
Source: Diegelmann, S., et al. The CApillary FEeder Assay Measures Food Intake in Drosophila melanogaster. J. Vis. Exp. (2017).
Copyright © 2025 MyJoVE Corporation. All rights reserved