存在稳定的分子是因为共价键将原子结合在一起。共价键的强度通过使其断裂所需的能量,即分离键合原子所需的能量来测量。分离任何一对键合原子都需要能量—键越强,打破它所需的能量就越大。
打破一摩尔气态分子中特定共价键所需的能量称为键能或键离解能。双原子分子的键能D X&Y 定义为吸热反应的标准焓变:
例如,纯共价H–H键的键能DH–H为436 kJ / mol的H–H键断裂:
具有三个或更多个原子的分子具有两个或更多个键。这种分子中所有键能的总和等于吸热反应的标准焓变,该吸热反应破坏了分子中的所有键。例如,CH 4 中四个C–H键能之和为1660 kJ,等于反应的标准焓变:
平均C–H键能DC–H为1660/4 = 415 kJ / mol,因为每摩尔反应有4摩尔C–H键断裂。尽管四个C–H键在原始分子中是等效的,但它们并不需要相同的能量才能断裂。一旦第一个键断裂(需要439 kJ / mol),其余键就更容易断裂。 415 kJ / mol的值是平均值,不是打破任何一个键所需的精确值。
两个原子之间的键强度随着键中电子对数量的增加而增加。通常,随着粘结强度的增加,粘结长度减小。因此,三键比相同两个原子之间的双键强和短。同样,双键比相同两个原子之间的单键强和短。当一个原子与基团中的各个原子键合时,键合强度通常会随着我们向下移动基团而降低。例如,C–F为439 kJ / mol,C Cl为330 kJ / mol,C–Br为275 kJ / mol。
键能可用于计算反应的近似焓变,也称为键焓,其中没有形成焓。这种类型的计算还将告诉反应是放热的还是吸热的。当产物中的键强于反应物中的键时,发生放热反应(ΔH为负,产生热量)。当产物中的键比反应物中的键弱时,就会发生吸热反应(ΔH为正,吸收热量)。
化学反应的焓变ΔH大约等于打破反应物中所有键所需的能量之和(能量“ in”,正号)加上产品中所有键均形成时释放的能量(能量“出”负号)。可以通过以下方式用数学方式表示:
在此表达式中,符号Ʃ表示“和”。 D表示键能,单位为kJ / mol,始终为正数。键能取决于特定键是单键,双键还是三键。因此,在以这种方式计算焓时,重要的是考虑所有反应物和产物中的键合。由于D值通常是许多不同分子中一种键的平均值,因此该计算提供了反应焓的粗略估计,而不是精确值。
请考虑以下反应:
或
这些多余的能量以热量的形式释放出来,因此反应是放热的。
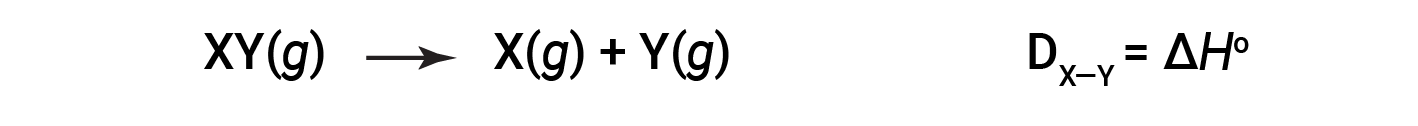


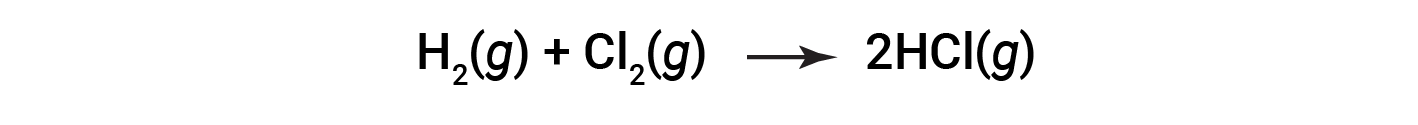
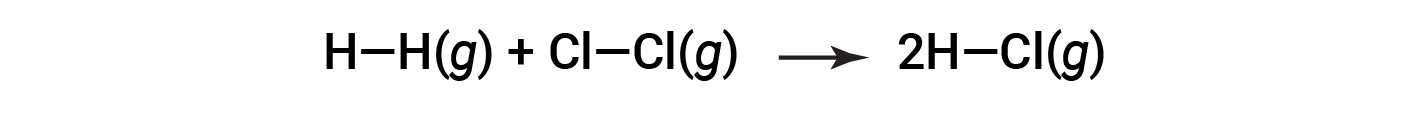

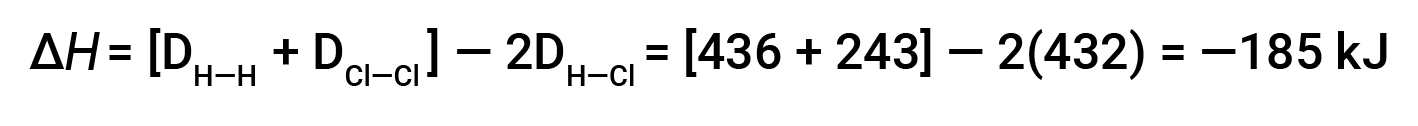
来自章节 9:

Now Playing
9.13 : 键能和键长
化学键合:分子几何学和成键理论
24.4K Views

9.1 : 化学键的类型
化学键合:分子几何学和成键理论
73.3K Views

9.2 : 路易斯符号和八偶体规则
化学键合:分子几何学和成键理论
58.3K Views

9.3 : 离子键和电子转移
化学键合:分子几何学和成键理论
37.4K Views

9.4 : 玻恩-哈伯循环
化学键合:分子几何学和成键理论
21.0K Views

9.5 : 晶格能量的趋势:离子大小和电荷
化学键合:分子几何学和成键理论
23.3K Views

9.6 : 共价键和路易斯结构
化学键合:分子几何学和成键理论
44.5K Views

9.7 : 电负性
化学键合:分子几何学和成键理论
62.7K Views

9.8 : 键极性、偶极矩和离子性百分比
化学键合:分子几何学和成键理论
27.9K Views

9.9 : 分子化合物和多原子离子的路易斯结构
化学键合:分子几何学和成键理论
33.7K Views

9.10 : 共振
化学键合:分子几何学和成键理论
49.3K Views

9.11 : 形式电荷
化学键合:分子几何学和成键理论
31.7K Views

9.12 : 八偶体规则的例外情况
化学键合:分子几何学和成键理论
26.5K Views

9.14 : 金属中的键
化学键合:分子几何学和成键理论
43.4K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。