Method Article
新的治疗肠道病变的疗效,使用活体小鼠的串行内镜成像的非侵入性评估
摘要
We describe methods for longitudinal monitoring of the efficacy of therapeutics for the treatment of colonic pathologies in mice using a rigid endoscope. This protocol can be readily used for the characterization of the therapeutic response of an individual tumor in live mice and also for monitoring potential disease relapse.
摘要
Animal models of inflammatory bowel disease (IBD) and colorectal cancer (CRC) have provided significant insight into the cell intrinsic and extrinsic mechanisms that contribute to the onset and progression of intestinal diseases. The identification of new molecules that promote these pathologies has led to a flurry of activity focused on the development of potential new therapies to inhibit their function. As a result, various pre-clinical mouse models with an intact immune system and stromal microenvironment are now heavily used. Here we describe three experimental protocols to test the efficacy of new therapeutics in pre-clinical models of (1) acute mucosal damage, (2) chronic colitis and/or colitis-associated colon cancer, and (3) sporadic colorectal cancer. We also outline procedures for serial endoscopic examination that can be used to document the therapeutic response of an individual tumor and to monitor the health of individual mice. These protocols provide complementary experimental platforms to test the effectiveness of therapeutic compounds shown to be well tolerated by mice.
引言
结肠直肠癌(CRC)是恶性全世界1的4 个最常见的原因。尽管我们对这个疾病的家族基础上的理解显著的进步,遗传易感性不仅有助于对CRC的情况下2〜20%。其余都归因于大量的外在因素和环境因素,包括慢性炎症。在人类中,慢性炎症和结肠直肠癌之间的关联是溃疡性结肠炎(UC)的患者,谁具有显影结肠炎相关结肠癌(CAC)的更大的风险明显,取决于炎性疾病3的持续时间,程度和严重性-5。因此,新的治疗方法是在发展中,以控制的免疫反应和相关的生产的生长促进因子的炎症肿瘤微环境6-8。有适当的临床前动物模型中增加的需求来表征的治疗功效这些药物对发育和疾病的进展。
小鼠模型已经明确证实,炎性微环境有助于CRC进展,即使在没有明显的炎症9,10。这些模型包括使用多糖葡聚糖硫酸钠(DSS)的,在小鼠的饮用水提供到上皮损伤和急性和慢性炎性肠疾病(IBD)的11,12建模。虽然通过该DSS诱导粘膜损伤和结肠炎的机理还不完全清楚,一些研究表明,DSS抑制细胞的逆转录酶和核糖核酸酶活动的细胞内,或促进纳米脂质复合物,与结肠膜导致上皮损伤熔合形成13,14。修改标准DSS的模型还提供显著洞察由结肠上皮细胞维持组织稳态和单组机制晚黏膜免疫反应15。
氧化偶氮甲烷(AOM)的腹腔内给药单独,或与DSS的结合,提供了一个模式用于检查的体细胞突变之间的相互作用,在上皮粘膜和炎症和基质微环境16,17。 AOM是致癌物质1,2-二甲基肼(DMH)不直接导致DNA突变的代谢物。相反,AOM水解methylazoxymethanol(MAM)由细胞色素异构体CYP2E1在肝脏,在那里MAM缀合有葡糖醛酸,然后通过胆汁分泌物18输送到肠。据认为,该细菌β葡糖醛酸酶有助于MAM造成DNA烷基化的降解和突变的上皮细胞19的积累。大多数的AOM诱导的结肠肿瘤窝藏错义突变的基因中的编码β连环蛋白,渲染蛋白耐蛋白酶degradati上,这会导致经典Wnt信号通路20的异常活化。当AOM的活性是结合由DSS诱发的粘膜损伤,随后的伤口愈合反应创建一个微环境有利于的诱变上皮的生长和扩增。在此模型中的一个变型中,AOM的单独一段数周重复给药可用于模拟散发性结直肠癌,在没有DSS诱导的结肠炎的10,17。这两种模式的免费提供实验设置分别进行研究的CAC和零星的CRC,这两者都与一个促炎症肿瘤微环境10相关联。
利用小鼠序列内窥镜是由贝克尔和他的同事21首创,并允许结肠炎和肿瘤进展的纵向监测。在这里,我们提供了三个临床前的协议的基础上DSS诱发的粘膜损伤和/或AOM介导的涂铁道部感应可重复诱导特异性结肠病变。第一个协议描述诱发急性胃黏膜损伤响应DSS管理引发许多与IBD相关的病理特点。所述第二协议是基于DSS给药三个连续的周期,以模仿炎症在IBD患者中观察到的耀斑,并且可以进行结合AOM诱导突变。最终的协议是基于AOM诱导零星上皮突变。对于每一个这些协议中,我们在相关标准程序展开以包括我们已经开发并监控的新药物的疗效的预防和治疗性干预的方法。
研究方案
医学研究动物伦理委员会的沃尔特伊丽莎研究所厅批准每一个在这些协议中描述的过程。
1.准备实验小鼠
- 构成的至少4性别实验队列匹配6-8周龄C57BL / 6小鼠(M.家鼠 ),它们孕育和容纳在相同的特定的无病原体(SPF)的动物设施/室和设置有蒸压食物和水。使用雌性小鼠将允许共壳体小鼠从不同的线路,限制框号,以及降低笼到笼的变化。
- 确保小鼠是至少6周龄,使用3.0毫米直径的内窥镜护套内窥镜程序。
- 使用的小鼠相同的遗传背景,并且在可能的情况,从存储在同一机架,以减少与肠道菌群的变化相关联的笼中。在动物设施之间菌群变化应当考虑到磨片Ñ 确定适当的对照小鼠和基因型每个实验22。
- 马克小鼠耳/趾剪报,纹身,或类似的允许,便于识别。
- 称重在第0天的小鼠,以确定基线实验权重。
- 进行胃镜检查(见第5)小鼠第0天,记录基线结肠表型。
2,临床前试验中的上皮损伤和急性结肠炎型号
- 制备的治疗剂的溶液,以进行测试。对于通过口服管饲法(PO)递送治疗剂,制备溶液,以100微升的最大体积。通过腹膜内(IP)注射递送治疗剂,制备溶液,以200微升的最大体积。
注:在长时间重复IP或口服给药可能导致改变鼠标的行为。交替腹腔注射部位,以避免长时间的不适。对于口服给药,提供小鼠autoclaved葵花籽作为一个'治疗',以减少与灌胃过程中的不良联想。 - 在第1天,施用的利息(步骤2.1的方法制备)和适当的载体对照的治疗。在提供的例子中( 图6A),5微克重组人(rh)下iInterleukin素(IL)-11的蛋白质溶于200微升磷酸盐缓冲盐水(PBS),并施用IP
- 确定施用,这将依赖于建立该正被测试的治疗试剂的药物动力学分布的时间和频率。 图1概括了使用每天两次腹腔注射在实验的过程中预防性治疗。
- 监控,包括粪便稠度和血液的存在,并每日称重每只小鼠。在治疗的日子里,称量应与治疗药物的给药重合,以减少应力所引起的反复操作时的小鼠。
- 在第3天,制备2.5%(2.5克/ 100毫升)中常规地由动物设施提供给小鼠的饮用水的DSS溶液。大约5毫升/小鼠/的DSS溶液一天所需的实验。如果动物设施的环境温度波动的DSS水的消耗可能会改变。
注:DSS是具有刺激性,并应按照MSDS的指令来进行处理。 - 提供新鲜DSS的小鼠自由采食清水瓶连续5天。在5日的晚上,提供正常的饮用水除泥状食物颗粒和小皮氏培养皿(100克食物颗粒/ 10克蛋白质摇/ 10毫升的水)提供的蛋白质补充。这可以防止脱水,并协助最大限度地减少重症减肥。
- 安乐死小鼠二氧化碳中毒和收集组织进行后续组织学分析的第8天,或早晨,当小鼠经历≥15%减重超过三Consecutive天,以先到为准。
注:调整之间1-4%的DSS剂量(重量/体积)根据动物设施的菌群和批次的DSS。进行,常规批量测试,以建立相应的DSS剂量和规避批次到批次的变化。合适的剂量应根据瘦身(原始重量的不大于15%),并确认结肠炎组织病理学。
3.临床前试验在慢性结肠炎或结肠炎相关的癌症模型
- 对于慢性结肠炎模型开始从步骤3.7。
- 对于结肠炎相关癌症(CAC)的模式,在第1天,以10毫克/公斤(W / W)氧化偶氮甲烷(AOM,250微升,IP)注射各小鼠。
注:AOM是一种致癌物质,并应按照MSDS的指令来进行处理。 - AOM的储备溶液制备为10毫克/毫升的等分试样并储存在-20℃。对注射的当天,AOM的原液解冻,并稀释至1毫克/米升的PBS。应避免反复冷冻和AOM的原液解冻。
- AOM的剂量可以8-12毫克/公斤之间调整。常规批量测试需要建立相应的AOM剂量以规避毒性小鼠由于批次到批次的变化。
- 在第1-7天以下的AOM施用权重将是稳定的,因为这个原因重量监控可以第1-7天期间被省略,以避免处理的动物,这将排泄的代谢物的细胞毒性在其粪便。
- 在第7天全部寝具需要下列细胞毒性程序来改变。一旦被褥变化完成后,该动物不再需要的细胞毒性的处理程序,因为它们将不再被排泄细胞毒性代谢物。
- 在第8天,制备2.5%(2.5克/ 100毫升)的DSS溶液(如在步骤2.5中描述),并提供给小鼠自由进食。
- 每天称量小鼠在整个实验过程和监测疾病的迹象,包括竖起福R,拱起,便血和减少运动。
- 第13天,取出DSS解决方案,并提供小鼠与正常饮水,直到28日8-28天构成慢性结肠炎/ CAC协议的一个"周期"。
- 在13-28天,当小鼠给予正常饮水,提供小鼠捣碎食物颗粒和小皮氏培养皿(100克食物颗粒/ 10克蛋白质摇/ 10毫升的水)提供的蛋白质补充。这可以防止脱水,并协助最大限度地减少重症减肥。
注意:当小鼠接受DSS含饮用水,以确保DSS消费一致性蛋白补充剂醪不应该提供的。 - 在20日进行的第二次内镜检查来监察个别鼠标的健康(见第5)。
- 在29天的重复周期DSS(周期2:2.5%(重量/体积)DSS天34-50天期间29-33和正常饮水时提供)。
- 在40日进行的THIRD内镜检查来监察个别鼠标的健康,并确定肿瘤负荷(见第5)。
- 如果没有肿瘤是可见的内镜40日,开始在天50-72第三DSS周期(周期3:2.5%(重量/体积)DSS天56-72天期间50-55和正常饮水时提供)。通常,肿瘤是由40天可见,因此,决策支持系统的第三周期可以省略。
- 一旦肿瘤变得可见通过内镜检查,分配给每个鼠标内窥镜肿瘤分数(见第5章 ),并指派个别小鼠同伙有类似的基线肿瘤负荷。
- 第42天(或当肿瘤是可见的),管理治疗化合物或相关的车辆控制(参见2.2节)。剂量的定时将依赖于治疗剂的药代动力学曲线。干预治疗的一个例子的小鼠腹腔注射每周三次,提供建立的肿瘤( 图2)。
- 执行电子ndoscopic考试每周一次以上的治疗性治疗的持续时间来监测肿瘤负荷。通常,4周治疗性治疗是足够观察客观治疗反应。以下戒烟治疗,小鼠的队列也可以通过内窥镜对肿瘤复发监测。
- 安乐死小鼠二氧化碳中毒和或当小鼠经历≥15%减重连续三天以上,以先到者为准收集组织进行生化和/或组织学分析72日的早晨。
4.临床前试验的散发性结直肠癌型号
- 在第1天,用10mg / kg的急性中耳炎(参见步骤3.2)注入每只小鼠的IP。 AOM的剂量可以8-12毫克/公斤之间调整。常规批量测试需要建立相应的AOM剂量以规避毒性小鼠由于批次到批次的变化。
- 重复注射,每周的第一天对于以下5个周( 图3)。小鼠应根据细胞毒性安全程序为整个6周处理。
- 权衡对AOM注射当天每只小鼠以减少痛苦而引起反复操作时的小鼠。
注:AOM是一种致癌物质,并应按照MSDS的指令来进行处理。 - 所有的动物应根据细胞毒性安全程序的6周需要这种协议AOM腹腔注射处理。
- 在8周进行内镜检查,以监测为新兴的肿瘤(见第5)。内镜检查应进行每两个星期,直到肿瘤变得可见。通常,野生型C57BL / 6动物开始的AOM的初始给药后发展的肿瘤约40周。
- 一旦肿瘤变得可见通过内镜检查,分配给每个鼠标内窥镜肿瘤分数(见第5章),并指派个别小鼠类似队列之前治疗的肿瘤开始负担。
- 在40周(或当肿瘤是第一个可见的),管理治疗化合物或相关车辆控制。
- 剂量的定时将依赖于治疗剂的药代动力学特性(参见步骤2.1)。干预治疗的一个例子的小鼠腹腔注射每周3次,用被设置已建立的肿瘤( 图3)。
- 用于治疗性治疗周期的持续时间来监测肿瘤负荷进行内窥镜检查每周。通常,4周治疗性治疗是足够的客观治疗反应。治疗后停止,小鼠的队列也可以通过内窥镜肿瘤复发监测。
- 安乐死的小鼠用CO 2中毒并在45周,或以下4周治疗收集组织用于结肠和肿瘤的生化和/或组织学分析。
- 该内窥镜设备应当根据标准程序16进行组装。
- 消毒和清洁,用70%的乙醇或抗细菌润滑剂的内窥镜探针。
- 画可以使用笔记本或台式计算机和标准的媒体软件,如iMovie中被记录。一个标准的计算机监视器,而不是医用级监视器,足以显现内镜程序( 图4)在结肠。
- 麻醉5-6只小鼠的组同时与在100%O 2 3%异氟醚的腔室以0.2-0.4升/分的速率。一旦麻醉的小鼠,它是由脚趾捏确认;在异氟烷水平应改变以0.5-2%以进行维护。
- 从室内除去个别的鼠标,并把它腹侧了它的头固定在鼻锥。充分麻醉应监视和后腿应调整,使它们有stretched淘汰落后的鼠标。
- 放置在老鼠的眼睛兽医药膏,以防止干燥。
- 持在那里它满足下脊椎清楚地揭示了肛门鼠标的尾部。插入硬性内窥镜鞘成水的烧杯中,并调整气流,以允许其中一个小气泡的释放的时间。
- 气流将允许结肠膨胀。如果气流太强,空气将被泵入小鼠的胃。没有额外的润滑是必需的。
- 小心地将硬性内窥镜套入直肠。通常,内窥镜能插入多达3厘米,在这一点,结肠小鼠曲线和不能访问的硬性内窥镜。
- 在内窥镜检查程序的常见困难是获得结肠的内腔堵塞因粪便物。通过之前的程序轻轻按摩小鼠腹部鼓励排便,或精心操纵内窥镜周围的FECA避免这种在手术过程中升的问题。蠕动,也可能发生,在此期间,内窥镜应在适当的位置被保持到结肠肌肉松弛。
- 启动视频录制在内窥镜程序的任何阶段。病的得分可以由有经验的助手在手术期间被记录,或从视频文件稍后的时间点。
- 继内镜检查,返回老鼠笼子,并从麻醉恢复过程中监视他们。动物一般都是清醒和移动在2分钟之后删除所管理的异氟醚鼻锥。
- 确保这些程序是由经验丰富的科学家进行的。每个内窥镜程序将每只小鼠约2分钟。
6.疾病评分
- 并监控结肠炎,得分视频的小鼠内镜结肠炎的严重程度(MEICS)结肠壁由透明表示的厚度指数16,其(i)中记载的改变,(ⅱ)大便稠度,(ⅲ)血管完整性和存在,(iv)对出现在粒状时尚溃疡和再生区,(v)的出血表示由纤维蛋白沉积( 图5a)。
- 监测肿瘤的负担,进球视频用于肿瘤的发病率和大小。肿瘤大小通过由肿瘤16( 图5b)所占据的结肠腔的直径来决定。它通常使用既结肠炎和肿瘤评分参数为单个鼠标。用内窥镜,个体肿瘤和结肠黏膜的健康可随时间被监测,提供一个读出的新的治疗剂的成功在单个动物。
- (可选)静止图像可以从视频中提取的一代的代表性人物( 图6)。
结果
瘦身用作标准参数以监视与结肠炎,这是经常用于监测小鼠的总体健康相关的疾病。动物一般保持自己的体重,而DSS-含有水的管理,只有开始减肥瘦身的时候都恢复正常饮水。可接受的减肥参数应建立符合该机构的动物伦理委员会。为了防止与长期腹泻相关的脱水,使用例行提供醪(食物颗粒磨碎并与饮用水混合),补充有除了正常饮用水的蛋白质抖动。
作为替代麻醉,氯胺酮/赛拉嗪或类似试剂都可以使用,如果异氟烷设备不可用。由于内窥镜的刚性性质,这些过程只允许的最远端3厘米的小鼠结肠的可视化。新的内窥镜用额外的功能(包括柔性和荧光)可根据实验的需要。然而,由于决策支持系统的有害作用主要限于小鼠的远端结肠,用较温和的病理学在中间结肠观察到的,硬性内窥镜不妨碍监测个体小鼠的粘膜健康。虽然我们描述了一个协议,用于预防治疗急性DSS诱发结肠炎,这个协议可以很容易地修改,以测试干预治疗策略。旨在减轻上皮损伤和结肠炎的药物的功效可以在个体小鼠纵向进行监测,并根据所描述的评分参数( 图5)定量。这是有利的,在传统的实验设计,其中实验方案中所需要的小鼠在特定时间点的剔除,而不允许该疾病响应于治疗随时间的表征。
吨">在人体临床研究已经强调了在肿瘤如何不同的个体患者响应处理相当大的变化。在此处所描述的程序,提供给监控总体肿瘤负荷,以及个别肿瘤在过程中治疗响应的装置的实验,以考虑所概述的癌症模型中的干预协议不考虑到肿瘤发生疗法的效果,这是很重要的。预防性协议,与之前当肿瘤变得内镜可见提供了治疗,需要获得此信息。这里概述的协议提供上的上进展的治疗作用的信息从个人肿瘤(肿瘤大小测量)。肿瘤消退,也可以通过在可见的肿瘤数量的减少来表示。G"/>
图1:监视预防性治疗疗法的疗效的急性结肠损伤模型中的实验方案(一)需要8天,从开始到完成。治疗从第1天服用(二)预防性治疗。 DSS是在饮用水(c)从所述的实验方案的第3天提供的。内窥镜进行(D)监测动物的疾病进展。建议的时间点包括第0天(未处理)和第2天(健康监控),5日和8(以确定疾病负担)。在实验终止8.日(e)该早晨在野生型小鼠(F)的疾病的增加随着时间的进展。
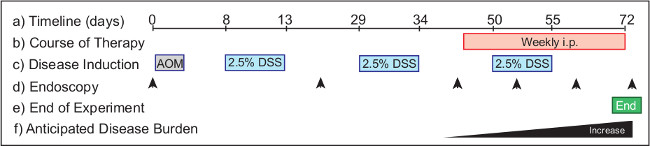
图2:监控介入治疗的疗效模型Ø˚F结肠炎相关癌症的实验方案(一)需要72天从开始到结束。治疗剂(b)的从46天给予小鼠与已建立的肿瘤干预治疗。 AOM(c)的注入在第1天,和DSS设置在饮用水过度的实验方案的三个周期的过程中,开始在8内窥镜(d)中进行监测,动物的疾病进展日。建议时间点包括第0天(未处理),第20天(健康监控),和40天(至动物组根据肿瘤负荷)。内窥镜进行每周在治疗的过程中,以监测疾病的结果。实验(五)终止72日上午在野生型小鼠(六)肿瘤负荷增加,从40日起实施。

图3:监测介入治疗的疗效的自发结直肠癌模型中的实验方案(一),需要>50周从开始到完成。治疗剂(b)的施用给小鼠已建立的肿瘤干预治疗。 AOM(c)的注入在第1天,此后,连续6周注射在实验方案的过程中。内镜(d)中进行监测,动物的疾病进展。建议的时间点包括第0天(未处理)和第8周(肿瘤监测)和双周有后(建立肿瘤负荷)。内窥镜进行每周以上治疗过程中监测治疗结果。在实验(e)的终止在周50.在野生型小鼠(F)的肿瘤负荷增加从40周开始。 P>
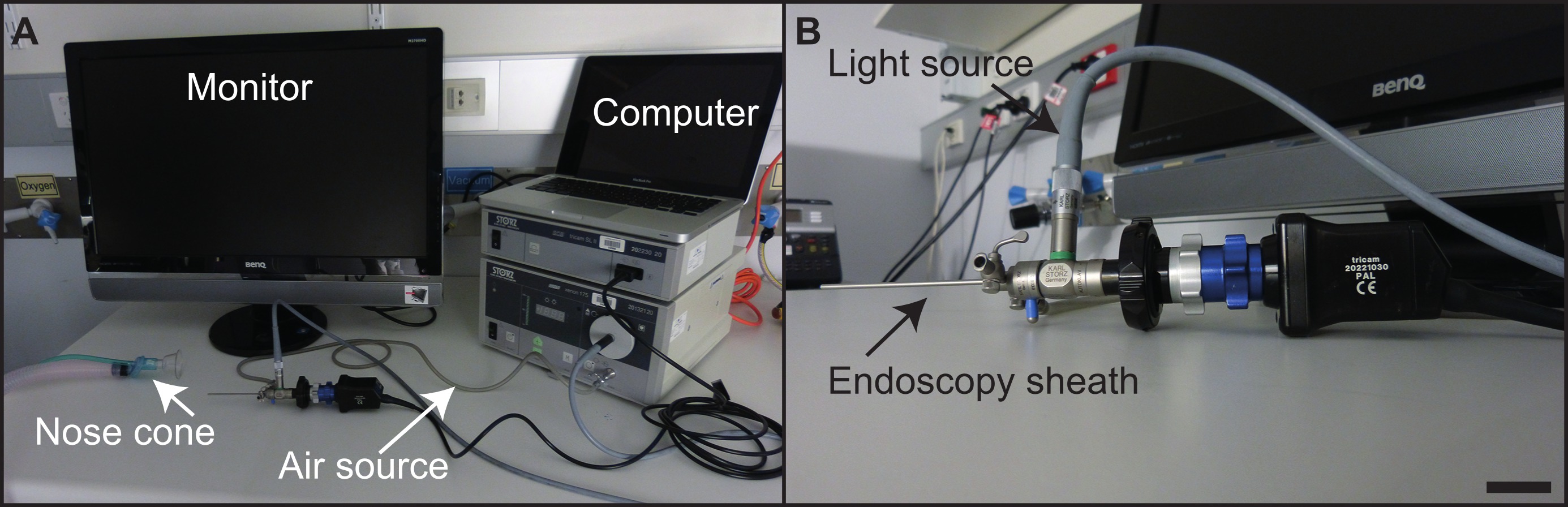
图4:设备建立的实验设置(a)在所述内窥镜部,与单件设备的指示。的硬性内窥镜(b)中 ,与个别组分表示。比例尺= 2.5公分。 请点击此处查看该图的放大版本。
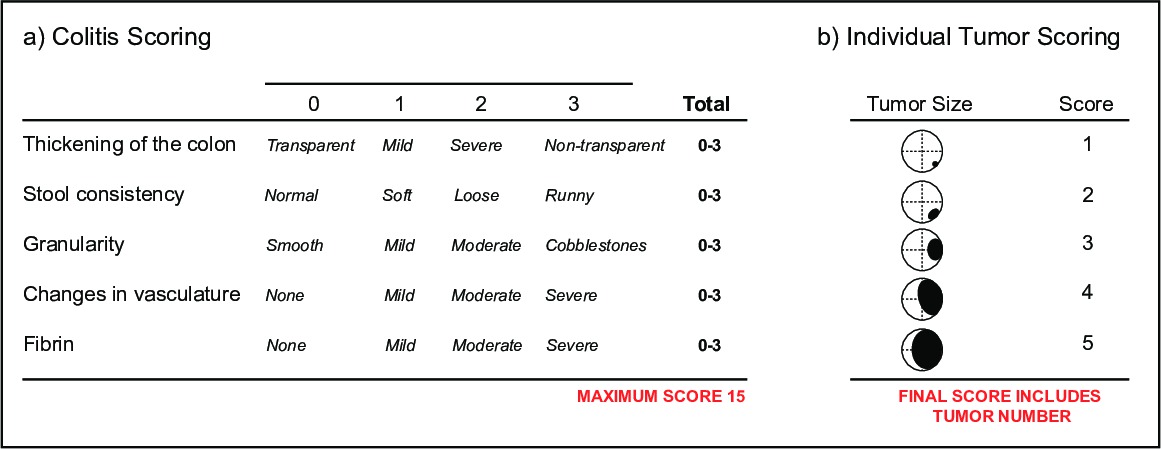
图5:得分病参数经胃镜大纲(一)鼠内镜结肠炎严重程度(MEICS)指数。个别肿瘤得分参数的大纲(二)。ES / ftp_upload / 52383 / 52383fig5highres.jpg"目标="_空白">点击此处查看该图的放大版本。
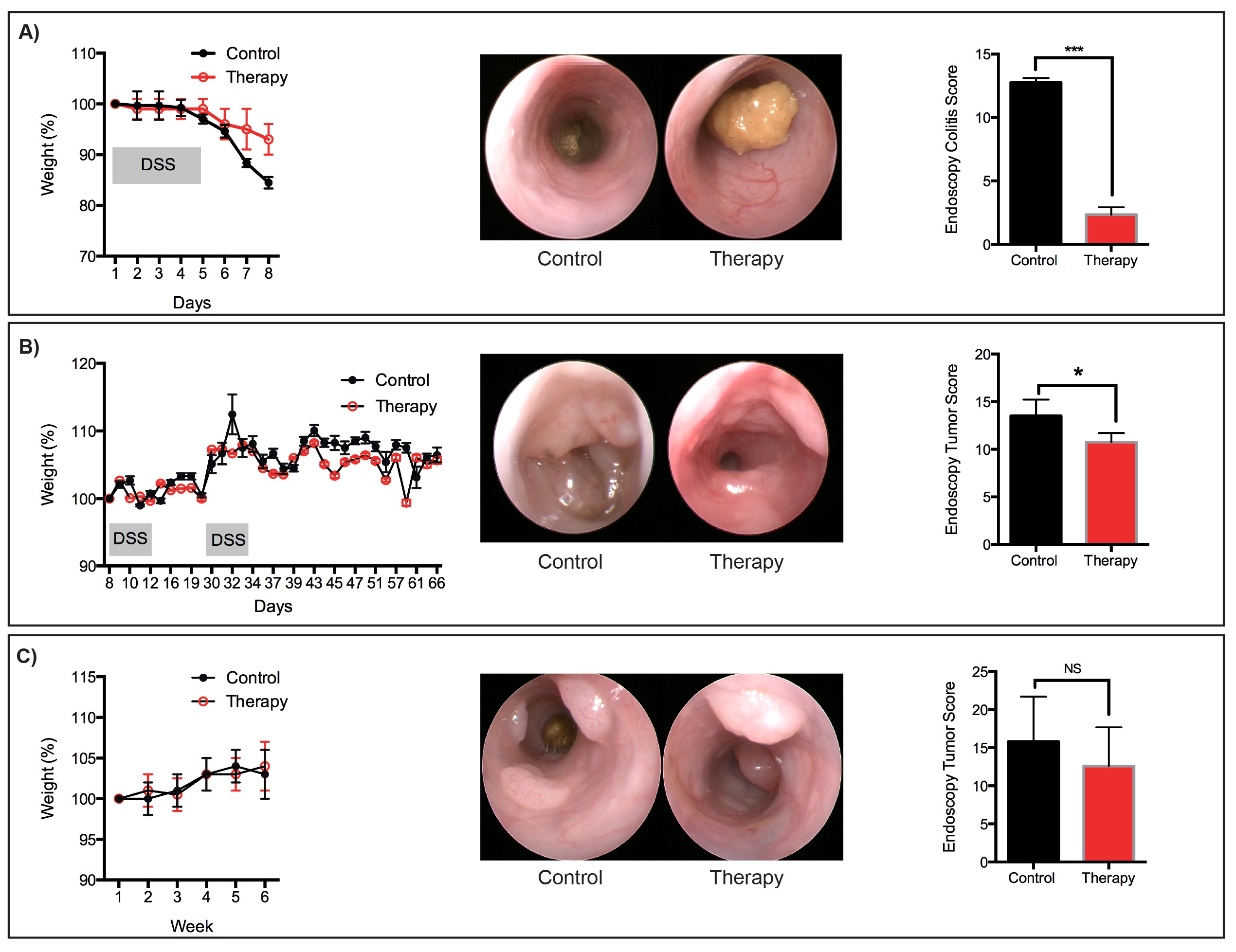
图6:代表理疗服务代表减肥,内窥镜图像和分数为:(一)急性DSS诱导粘膜损伤(二)肿瘤,发达国家继AOM / DSS协议(三)肿瘤的发展遵循的顺序。 AOM协议。 N = 3只小鼠每组。 * P <0.05,*** P <0.001(学生t检验)。 请点击此处查看该图的放大版本。
讨论
The three protocols that are described outline methods of reliable and reproducible induction of colonic disease pathology in mice. When combined with routine endoscopic monitoring and the intervention strategies outlined here, these protocols will provide powerful pre-clinical insight into the efficacy of therapeutics. Our laboratories routinely use all of these protocols to monitor the success of novel therapeutics10,23,24.
There are a number of considerations when choosing a pre-clinical animal model to test new therapeutics. These include relevance of the model to the human disease, and the contribution of the tumor microenvironment to the proposed action of the therapeutic target. Here we provide three protocols for therapeutic intervention in established intestinal disease models. These models are reproducible and the delivery of reagents to induce disease is easy to manage. Importantly, the models are highly relevant to multiple facets and stages of colitis onset, and of tumor initiation and progression. Researchers should take into consideration the genetic background of the mouse strains used when designing experiments, as the susceptibility to disease induced by DSS and/or AOM can vary considerably25. In addition, different microbial communities may have different metabolic capacities in the context of AOM, which is metabolized by bacteria. We caution against using different cohorts of mice that were born into different animal facilities (including commercial vendors) in a single experiment. Similarly, the different microflora in mice used from different facilities may elicit different host responses to DSS-induced epithelial barrier damage11. Moreover, the appropriate analysis of tissue (for example RNA purification) should also be considered, since the ability of DSS to inhibit reverse transcriptase will impact on subsequent molecular analysis26,27.
Mouse endoscopy is a cutting edge technique to repeatedly monitor disease onset and progression in an individual mouse. The ability to record videos and extract still images permits easy monitoring of multiple disease parameters and tumors. In addition to improving animal welfare, endoscopic monitoring also reduces the need for multiple cohorts of experimental mice, which traditionally were culled at different time-points to track disease outcome. The MEICS scoring system is not a substitute for histopathological analysis, but provides an alternative means to monitor animal health and mucosal damage in live mice. Mouse endoscopy is a specialized laboratory technique, and all procedures should be performed by trained personnel to ensure appropriate manipulation and handling of the mice, as well as to provide consistent quality in the images used for disease scoring. In the hands of qualified personal, we have found that endoscopy induces little or no damage to the tumors that would cause intra-tumoral bleeding. For the therapeutic protocols outlined, we consider endoscopy highly advantageous, since it provides a way to determine the initial tumor burden, and allows us to group cohorts of animals with similar tumor burdens prior to the administration of a therapeutic drug. Sequential monitoring of the mice enables researchers to determine the efficacy of novel therapies early on, with the option of terminating unsuccessful experiments in a timely manner.
As our understanding of inflammatory bowel disease and colorectal cancers advance, new targets for therapy will be identified. Appropriate animal models will be integral to ensuring that the most promising new therapies are moved towards clinical trials.
披露声明
The authors have nothing to disclose.
致谢
We would like to thank CSL Ltd. for supporting the purchase of the endoscopy equipment. The research in the laboratory of ME is supported by the Ludwig Institute for Cancer Research, and the laboratories of TP and ME are supported by the Victorian State Government Operational Infrastructure Support and the National Health and Medical Research Council of Australia. ME is an NHMRC Senior Research Fellow.
材料
| Name | Company | Catalog Number | Comments |
| Dextran sulfate sodium (MW 36,000-50,000) | MP Biochemicals | 160110 | Requires batch testing. |
| Azoxymethane | Sigma | A5486-100MG | Requires batch testing. |
| Vanilla protein shake | N/A | N/A | Available from hospital pharmacies. |
| Isoflurane | PPC | M60303 | This is a restricted reagent, which should be stored under lock and key. |
| 70% ethanol | N/A | N/A | Standard lab reagent. |
| Coloview miniendoscopic system | |||
| Endovision Tricam | Karl Storz | 20212001-020 | |
| Xenon 175 light source with anti-fog pump | Karl Storz | 20134001 | |
| HOPKINS straight Forward Telescope | Karl Storz | 64301AA | |
| Endoscopic sheath (total diameter 3 mm) | Kalr Stroz | 61029C | |
| Fiber optic light cable | Kalr Stroz | 69495ND | |
| Computer and media player software | Apple | iMovie | |
| Scale | Any | Any scale suitable for weighing mice. |
参考文献
- Tenesa, A., Dunlop, M. G. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat Rev Genet. 10, 353-358 (2009).
- Rustgi, A. K. The genetics of hereditary colon cancer. Genes Dev. 21, 2525-2538 (2007).
- Rutter, M., et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 126, 451-459 (2004).
- Eaden, J. A., Abrams, K. R., Mayberry, J. F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 48, 526-535 (2001).
- Lakatos, P. L., Lakatos, L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 14, 3937-3947 (2008).
- Xiang, B., Snook, A. E., Magee, M. S., Waldman, S. A. Colorectal cancer immunotherapy. Discov Med. 15, 301-308 (2013).
- Feng, Q. Y., et al. Anti-EGFR and anti-VEGF agents: Important targeted therapies of colorectal liver metastases. World J Gastroenterol. 20, 4263-4275 (2014).
- Dinarello, C. A. Anti-inflammatory Agents: Present and Future. Cell. 140, 935-950 (2010).
- Grivennikov, S. I., et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 491, 254-258 (2012).
- Putoczki, T., Thiem, S., Loving, A., Busuttil, R. A., Wilson, N. A., Ziegler, P., Nguyen, P., Preaudet, A., Farid, R., Edwards, K., Boglev, Y., Luwor, R. B., Jarnicki, A. J., Horst, D., Boussioutas, A., Heath, J., Sieber, O., Nash, A., Greten, F., McKenzie, B. S., Ernst, M. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 24, 257-271 (2013).
- Perse, M., Cerar, A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012, 718617(2012).
- Rose, W. A. 2nd, Sakamoto, K., Leifer, C. A. Multifunctional role of dextran sulfate sodium for in vivo modeling of intestinal diseases. BMC Immunol. 13, 41(2012).
- Laroui, H., et al. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One. 7, e32084(2012).
- Miyazawa, F., Olijnyk, O. R., Tilley, C. J., Tamaoki, T. Interactions between dextran sulfate and Escherichia coli ribosomes. Biochim Biophys Acta. 145, 96-104 (1967).
- Peterson, L. W., Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 14, 141-153 (2014).
- Neufert, C., Becker, C., Neurath, M. F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2, 1998-2004 (2007).
- Schwitalla, S., et al. Loss of p53 in Enterocytes Generates an Inflammatory Microenvironment Enabling Invasion and Lymph Node Metastasis of Carcinogen-Induced Colorectal Tumors. Cancer Cell. 23, 93-106 (2013).
- Fiala, E. S. Investigations into the metabolism and mode of action of the colon carcinogens 1,2-dimethylhydrazine and azoxymethane. 40, 2436-2445 (1977).
- Chen, J., Huang, X. F. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol Ther. 8, 1313-1317 (2009).
- Bollrath, J., et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 15, 91-102 (2009).
- Becker, C., Fantini, M. C., Neurath, M. F. High resolution colonoscopy in live mice. Nat Protoc. 1, 2900-2904 (2006).
- Ivanov, I. I., et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 139, 485-498 (2009).
- Thiem, S., et al. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J Clin Invest. 123, 767-781 (2013).
- Stuart, E., et al. Therapeutic inhibition of Jak activity inhibits progression of gastrointestinal tumors in mice. Mol Cancer Ther. 13, 468-474 (2014).
- De Robertis, M., et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 10, 9(2011).
- Viennois, E., Chen, F., Laroui, H., Baker, M. T., Merlin, D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res Notes. 6, 350(2013).
- Kerr, T. A., et al. Dextran sodium sulfate inhibition of real-time polymerase chain reaction amplification: a poly-A purification solution. Inflamm Bowel Dis. 18, 344-348 (2012).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。