Chronic Social Defeat Stress in Early Adolescent Male Mice
In This Article
Summary
This protocol details how to perform 10 day chronic social defeat stress in early adolescent male mice to produce long-lasting social avoidance in adulthood in more than 60% of mice.
Abstract
Physical abuse and trauma in childhood is reported in as many as 1 in 7 children and is a major risk factor for the development of psychiatric diseases, such as Post-Traumatic Stress Disorder (PTSD), in adolescence and adulthood. Hallmark behavioral symptoms of PTSD include avoidance of cues and contexts associated with trauma. The ability to model long-lasting changes in the brain due to early life trauma is critical to determine potential circuit and molecular targets for the modulation of resultant symptoms. This manuscript describes a protocol for modeling early life physical and psychological trauma in early adolescent male mice that produces socially avoidant behavior. Adolescent male mice are exposed to repeated aggressive encounters followed by overnight housing, which provides an added dimension of psychological stress, with an adult male aggressor every day for 10 days. Repeated social defeat paired with overnight housing by the aggressor in early adolescence produces robust social avoidance behavior that lasts throughout adulthood. Social avoidance can be readily quantified in early adolescents, adolescents, and adults using open field social interaction testing. Early adolescent social defeat robustly produces more than 60% susceptible animals in adulthood.
Introduction
As many as 1 in 7 children experience childhood abuse. Along with greatly increasing the risk for development of psychiatric diseases such as PTSD and other fear-related disorders in adulthood, this early life experience has a profound impact on brain anatomy and connectivity1,2. Early life physical abuse has recently been found to delay circuit maturation while early life neglect has found to accelerate it3,4, necessitating the development of models of early life physical trauma in addition to those of neglect such as maternal separation (MS)5 and limited bedding and nesting (LBN)6. Additionally, MS and LBN models are carried out in infant animals before weaning. As trauma during middle childhood (ages 10-12) is especially linked to susceptibility to PTSD and psychiatric disorders later in life7, the ability to model early life stress at developmental stages outside of infancy is critical.
To develop a model of early life trauma with behavioral effects that last into adulthood, this 10 day early adolescent chronic social defeat stress (eaCSDS) protocol was designed with modifications of a traditional adult chronic social defeat stress model8,9,10. The traditional protocol has been unsuccessful in maintaining the long-term aggression towards adolescents that is needed to successfully perform 10 consecutive days of defeat. In this protocol, early adolescent C57/B6J males (4 weeks old, ~P30) are repeatedly defeated by a young (8-10 weeks) adult CFW male that has no breeding experience, a modification of the traditional use of retired CFW male breeders. Another key modification of the traditional protocol is that the aggression of the CFW males is heightened by 1 week of co-housing with an ovariectomized female. After this housing, CFW males rapidly and consistently attack adolescent males, negating the need for stimulus adult C57/B6J mice to increase aggression before defeating adolescent males. Another modification of the traditional adult model is the use of smaller cages (29 cm x 19 cm x 13 cm) during defeats and overnight housing.
Testing of susceptibility to defeat is performed using the open field social interaction test (OFSI). Mice that are susceptible to defeat are classified as animals that spend more time interacting with an empty cup than with a cup with a CFW male inside. The protocol described below produces greater than 60% susceptibility during testing in adolescence and adulthood.
Protocol
All experiments were approved by and performed in accordance with McLean Hospital Institutional Animal Care and Use Committee or VA Boston Institutional Animal Care and Use Committee guidelines. All mice were housed in a 12 h light/dark cycle and provided food and water ad libitum. See Figure 1 for a schematic overview of the protocol and the Table of Materials for details about all animals, materials, and equipment used in this protocol.
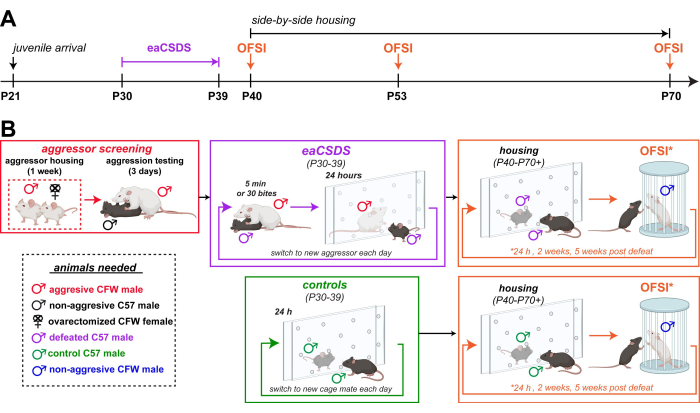
Figure 1: Schematic of experimental design for eaCSDS. (A) Timeline for C57 defeated and control animals. (B) Stages of eaCSDS presented in chronological order, beginning with aggressor screening and followed by repeated social stress for the experimental group, housing for both control and experimental groups, and finally, behavioral testing. Abbreviations: eaCSDS = early adolescent chronic social defeat stress; OFSI = open field social interaction. Please click here to view a larger version of this figure.
1. Screening aggressive CFW males
- Order C57 males such that they arrive at 3 weeks old from an external laboratory to ensure adequate time for aggressors to be screened and prepared for defeats before adolescents reach 4 weeks of age. Leave C57s group housed until 1 week after their arrival.
NOTE: Alternatively, animals can be bred inhouse. There have been no observed differences in defeat susceptibility based on whether mice are bred inhouse or shipped from an external laboratory. - Order adult (8-12 weeks, no breeding experience) CFW males and adult (>8 weeks) CFW ovariectomized (OVX) females. Order as many CFW males as C57s to be defeated, plus at least 50% extra in case aggression decreases over the defeat. For example, if defeating 10 adolescent C57s, order 15 CFW males and 15 CFW OVX females. At least 10 of the CFW males will become aggressive so, if required, use the other 5 non-aggressive males later as stimulus mice during OFSI.
NOTE: Nearly 80% of these CFW males will become aggressive after pair-housing. - One day after their arrival, pair-house CFW males with CFW OVX females (1 male CFW and 1 OVX female CFW per cage) for at least 1 week to allow for sexual experience and increase territorial aggression in the male.
NOTE: *CFWs can be pair housed until the defeat begins. - After 1 week, perform aggression testing:
- Take cages of pair housed CFWs to the testing room.
- Remove the CFW OVX female from the cage and place it in a clean, empty cage.
- Introduce the stimulus C57 male to cage with the CFW male for 1 min.
- Record the latency of attack. If an attack occurs, allow the attack to proceed until 1 min from the introduction of the stimulus C57 has elapsed.
- Remove the stimulus C57, place the CFW OVX female back in the cage with the CFW male, and return them to the housing room.
- Repeat this protocol for 2 more days. Use the mice showing an attack latency of <30 s for at least 2 days of screening for adolescent defeats. Continue housing aggressive CFW males with CFW OVX females until the first day of defeat.
NOTE: Though there have been no difficulties in eliciting aggression from CFW males after housing with OVX CFW females, removal of the ovaries stops the estrous cycle and can potentially influence sexual behavior, which could result in a lack of sexual experience in CFW males paired with CFW OVX females11,12. The use of females with ovarian ligation rather than ovariectomy is an option that may be used if difficulties in increasing CFW male aggression occur. - Group house CFW males that either do not attack or show an attack latency of >30 s on 2 or more days. Use these non-aggressive CFW males as stimulus animals during OFSI.
2. Handling control adolescent C57 males
- When adolescent C57s are 4 weeks old, weigh and record the weight of each animal.
- Separate animals into two equal cohorts (control and defeated), ensuring that the weights between the two cohorts are not significantly different.
- House one control adolescent C57 on one side of a plexiglass partition, with another control adolescent C57 on the other side of the partition (i.e., two control adolescents per cage).
- Store the control cages in a separate room from where the defeats will be taking place.
- For days 1-10, weigh and switch the control adolescent to the opposite cage side every day for 10 days total.
- On the eleventh day, house each control adolescent beside a different control adolescent with a plexiglass partition between the two for the remainder of the experiment.
NOTE: This is to ensure both defeat and control animals are housed beside an unfamiliar animal of the same treatment type during the post defeat phase.
3. Defeating experimental adolescent C57 males
- Weigh and record the weight of adolescent C57s to be defeated.
- Before beginning the defeat, take careful note of the following to maintain the health of the adolescents throughout defeats:
- Count each 'compound bite', or bites in which the resident bites and remains biting, then kicks the stomach of the adolescent, as two separate bites-these types of bites have higher bite force and can break the skin more easily. End the defeat immediately if adolescents receive more than two consecutive compound bites.
- End the defeat immediately if any blood or open wounds become visible. If wounds are observed after the defeat ends, apply antibiotic cream to the wound site. If any wounds larger than 4 mm in diameter occur, euthanize the mouse immediately.
- For adolescents that have open wounds, limit subsequent defeats to no more than five bites until the wound is no longer visible. After the wound has healed, return to the original defeat guidelines of 30 bites or 5 min per defeat.
- Remove the CFW OVX female from the cage with the resident CFW male and house it with other OVX females.
- Introduce the adolescent C57 to the cage of the resident CFW male and start the stopwatch.
- Record the aggressor identity, attack latency, number of bites (with clicker counter), and the time it takes for the adolescent to receive 30 bites.
- Remove the adolescent from the cage after 30 bites or 5 min, whichever occurs first.
- Place the plexiglass partition in the middle of the CFW male resident's cage, with the CFW resident on one side and the defeated adolescent on the other. Ensure that the partition is firmly in place and cannot be moved by the resident.
- In 24 h, repeat the defeat (steps 3.1-3.7) with a different CFW male aggressor.
- Repeat the defeat every 24 h for 10 days total, each day with a different aggressor.
- On the eleventh day, house the defeated adolescents beside the defeated adolescents, with a plexiglass partition between them for the duration of the experiment. Place the defeated adolescents in the same room as controls but on a separate shelf.
- Pair-house the CFW males with any CFW OVX females until the next set of defeats.
NOTE: The CFW OVX females used do not need to be the same as those previously paired with the CFW males.
4. Testing social avoidance after defeat with open field social interaction testing (OFSI)
- Habituate control and defeated animals to the testing room for 1 h.
- Record all sessions with animal tracking software. Place the wire cup against the left wall of the 44 x 44 x 44 cm acrylic arena at the midpoint of the wall and draw a 2 cm circular interaction zone around the cup.
NOTE: Ensure that the cup is placed in the same position every time to prevent changes in zones with each trial. - Place the C57 in the corner of the arena with an empty wire cup for 150 s.
- Remove the C57 from the arena and place it in a clean, empty cage. Place a nonaggressive, unfamiliar CFW adult male inside the wire cup, then place the wire cup inside the arena.
- Reintroduce the C57 to the arena with the CFW-containing wire cup for 150 s.
- Return both animals to their respective cages.
- Clean the arena with water, cleaning any areas with urine or feces with 70% ethanol, before testing the next C57.
- Calculate the length of time any body point is within the interaction zone for the session with the empty cup and the session with the CFW-containing cup. Divide the time spent in the interaction zone with the CFW-containing cup by the time spent in the interaction zone with the empty cup to produce the social interaction ratio.
- Perform the OFSI at any time after the 10 day defeat depending on the developmental window of interest. For eaCSDS, perform OFSI 1 day, 2 weeks, and 5 weeks after the date of the C57's final defeat.
- A social interaction ratio > 1 is considered typical for control animals. While most control animals will have a social interaction ratio above one, a small subset may have social interaction ratios < 1. For defeated animals, consider those with a social interaction ratio < 1 susceptible and those with a social interaction ratio ≥ 1 resilient.
Representative Results
eaCSDS produces robust reductions in social interaction in OFSI across a large number of animals (n = 50 animals) (Figure 2A-C); defeated animals show decreased measures of both social interaction ratio and raw social interaction time in comparison to controls at all time points tested. When tested 1 day after defeats, adolescent (~P40) defeated mice show a significant decrease in social interaction ratio and raw social interaction time from controls but no significant difference in raw time spent with an empty cup (control: 65.48 ± 4.99 s, defeat: 67.75 ± 3.86 s; two-tailed unpaired t-test, p = 0.72). This decrease in social interaction and raw social interaction time is greatest when animals are tested 2 weeks after defeat in late adolescence (~P54). Interestingly, at this time point, defeated mice show a significant increase in the raw time spent with an empty cup in addition to the significant decreases in social interaction score and raw social interaction time (controls: 51.58 ± 3.53 s, defeated: 68.62 ± 2.86 s; two-tailed unpaired t-test, p = 0.0003).
The decrease in social interaction and raw social interaction time when tested 5 weeks after defeat remains robust even in adulthood (~P70), but as seen 24 h after defeat, there is no significant difference in raw time spent with an empty cup (52.83 ± 10.13, 53.15 ± 3.48; two-tailed unpaired t-test, p = 0.97). Within a single cohort, there can be variability in the proportion of susceptibility. However, cohorts tested using this protocol have consistently produced susceptibility in adulthood in 60% or greater of defeated animals. A cohort with a higher percentage of susceptible animals (90%) in adulthood (Figure 2D-F) versus a cohort with a lower percentage of susceptible animals (71%) (Figure 2G-I) in adulthood is shown for reference. Summed heatmaps from the social interaction portion of OFSI are shown for 10 control and 10 defeated animals at 24 h, 2 weeks, and 5 weeks post defeat (Figure 3) to provide visual examples of how defeated mice consistently spend an increased amount of time in corners of the arena and a decreased amount of time inside the interaction zone.
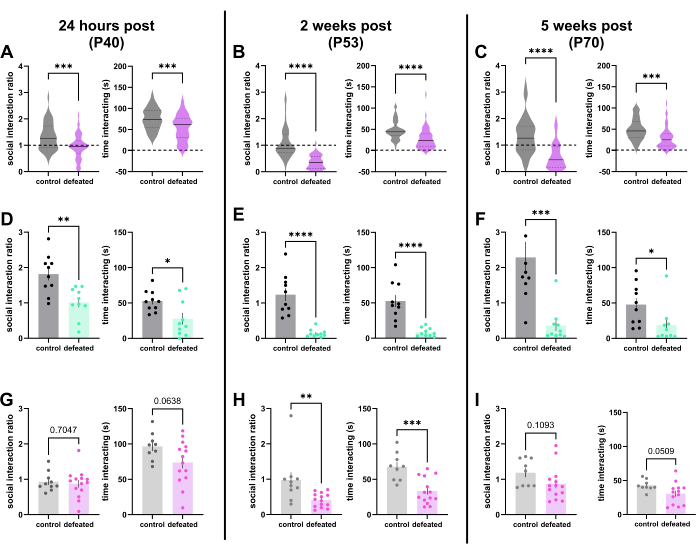
Figure 2: eaCSDS produces robust social avoidance in OFSI. (A-C) SI scores (left) and raw social interaction times (right) across 46 control and 50 defeat males (A) 1 day (~P40), (B) 2 weeks (~P54), and (C) 5 weeks (~P70) after defeat. (D-F) SI scores and raw social interaction times for an example cohort with a high percentage (90%) of susceptible animals in adulthood (as determined by average SI score of defeated adults) (n = 10 control and 10 defeated males) (D) 1 day, (E) 2 weeks, and (F) 5 weeks after defeat. (G-I) SI scores and raw social interaction times for an example cohort with a lower percentage (71%) of susceptible animals in adulthood (as determined by average SI score of defeated adults) (n = 10 control and 14 defeated males) (G) 1 day, (H) 2 weeks, and (I) 5 weeks after defeat. All comparisons were performed with unpaired two-tailed t-tests. **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. Abbreviations: eaCSDS = early adolescent chronic social defeat stress; OFSI = open field social interaction; SI = social interaction. Please click here to view a larger version of this figure.
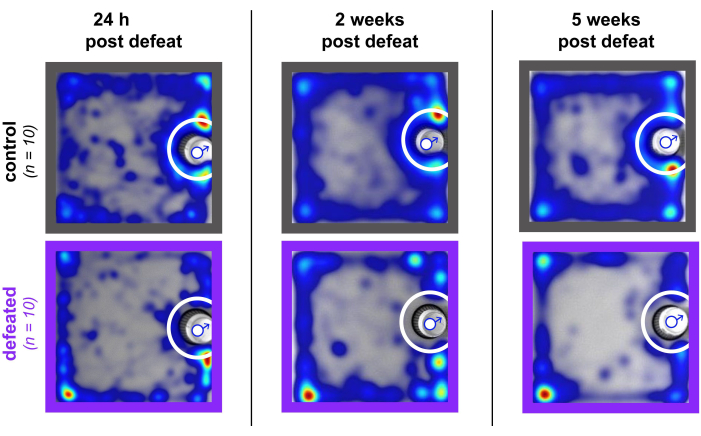
Figure 3: OFSI Heatmaps during social interaction in control and defeated animals. Heatmaps from the social interaction portion of OFSI testing (i.e., when nonaggressive CFW male is inside the cup) 24 h, 2 weeks, and 5 weeks after defeat. Note that each heatmap is summed over 10 animals (controls: n = 10 animals, grey; defeated: n = 10 animals, purple). Blue male symbol indicates location of cup with nonaggressive CFW male inside. White circle indicates location of interaction zone. Please click here to view a larger version of this figure.
Discussion
Early adolescent chronic social defeat stress (eaCSDS) results in robust social avoidance, as tested in open field social interaction (OFSI) tests, across the lifespan. This protocol was based on a traditional 10 day adult chronic social defeat stress model9 with several small, but key, modifications that differentiate it from traditional adult defeat and other early adolescent defeat protocols13,14,15. The first is the use of younger CFW males that have no breeding experience and are housed with females prior to defeat to increase territorial aggression. One possible explanation for the difficulty producing aggression towards adolescents in other protocols may be the use of retired breeders, which are typically much older and display lower levels of aggression than younger males. The second modification is the use of smaller cages in which mice are defeated and housed, which provides a smaller environment from which the intruder can avoid the resident both during the defeat and during housing after defeat. Finally, the number of bites is quantified to ensure that all mice receive consistent defeat experience.
This model is limited to late juvenile-early adolescent male mice. In its current form, this protocol has only tested defeats beginning at P26 at the youngest. It will be interesting in the future to examine the effects of this protocol on mice immediately after weaning, though the duration and number of bites during attacks may need to be limited given the smaller size of the mice. Notably, this protocol is for use in males, though preliminary evidence indicates it may also prove reliable for early adolescent female defeats with the modification of using female CFWs as aggressors.
Other models of early life stress typically target preweaning windows. Two of these models, maternal separation (MS) and limited bedding and nesting (LBN), are commonly used to induce early life stress before P10. Both MS and LBN affect cortisol levels and expression of corticotropin-releasing hormone (Crh)-related genes in pups16, but their effects on anxiety-like behavior are mixed17,18, and measures of adult social interaction in pups exposed to either paradigm are limited. LBN has been shown to reduce social interaction in adolescence19,20 and adulthood21 in rats, but, to our knowledge, its effects on social interaction in mice have not been reported. MS in C57/B6 mice has mixed effects on social interaction, with some groups reporting reduced social interaction22,23, while a more recent study reports an increase in social interaction24. In contrast to LBN and MS, this model of early adolescent defeat focuses on social interaction as a primary behavioral readout and targets stress to a later development period, which may account for the differences in social interaction across this model and MS and LBN models.
Early life physical abuse is incredibly prevalent in our society and often results in debilitating psychiatric conditions in adulthood. Despite its prevalence, the effects of early life abuse on adult neural circuits are not well understood, in part due to the lack of models of early adolescent physical abuse. The intention behind creating a protocol that facilitates consistent and robust social defeat in early adolescent males is to facilitate continued studies of the neural changes that arise from early life trauma.
Disclosures
KJR has performed scientific consultation for Bioxcel, Bionomics, Acer, Takeda, and Jazz Pharma; serves on Scientific Advisory Boards for Sage and the Brain Research Foundation; has received sponsored research support from Takeda, Brainsway, and Alto Neuroscience; and receives research funding from the NIH. All other authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgements
This work is supported by the National Institutes of Health (NIH) (Grant No. T32-MH125786 [to EH], Grant Nos. P50-MH115874 and R01-MH108665 [to KJR]), the Eric Dorris Memorial Research Award (to EH), and the McLean Frazier Fund (to KJR).
Materials
| Name | Company | Catalog Number | Comments |
| Quantity (for a cohort of 10 defeated and 10 control animals) | |||
| Arena, opaque | generic | --- | 1 Size: 44 x 44 x 44 cm |
| CFW male (8-10 weeks) | Charles River | 24 | 15 |
| CFW OVX female (8+ weeks) | Charles River | 24 | 15 |
| C57/B6J male (3 weeks) | Jackson Laboratories | 664 | 20 |
| C57/B6J male (6-7 weeks, stimulus mice) | Jackson Laboratories | 664 | 5 |
| Clicker counter | generic | --- | 1 |
| Home cages for housing during defeats | generic | --- | 15 (10 for defeated animals, 5 for control animals) Size: 29 x 19 x 13 cm |
| Plexiglass partitions | generic | --- | 15 Size: To fit 29 x 19 x 13 cm mouse cage |
| Stopwatch | generic | --- | 1 |
| Video camera | generic | --- | 1 Size: >15 frames per second capability |
| Wire cup | generic | --- | 1 Size: 10 cm diameter, 15 cm height |
References
- Colich, N. L., Rosen, M. L., Williams, E. S., McLaughlin, K. A. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychol Bull. 146 (9), 721-764 (2020).
- Lange, I., et al. Neurobehavioural mechanisms of threat generalization moderate the link between childhood maltreatment and psychopathology in emerging adulthood. J Psychiatry Neurosci. 44 (3), 185-194 (2019).
- McLaughlin, K. A., Sheridan, M. A., Lambert, H. K. Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 47, 578-591 (2014).
- Keding, T. J., et al. Differential patterns of delayed emotion circuit maturation in abused girls with and without internalizing psychopathology. Am J Psychiatry. 178 (11), 1026-1036 (2021).
- Tractenberg, S. G., et al. An overview of maternal separation effects on behavioural outcomes in mice: Evidence from a four-stage methodological systematic review. Neurosci Biobehav Rev. 68, 489-503 (2016).
- Gallo, M., et al. Limited bedding and nesting induces maternal behavior resembling both hypervigilance and abuse. Front Behav Neurosci. 13, 167 (2019).
- Stevens, J. S., van Rooij, S. J. H., Jovanovic, T. Developmental contributors to trauma response: the importance of sensitive periods, early environment, and sex differences. Curr Top Behav Neurosci. 38, 1-22 (2016).
- Berton, O., et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science (1979). 311 (5762), 864-868 (2006).
- Golden, S. A., Covington, H. E., Berton, O., Russo, S. J. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 6 (8), 1183-1191 (2011).
- Kudryavtseva, N. N., Bakshtanovskaya, I. V., Koryakina, L. A. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 38 (2), 315-320 (1991).
- Huijgens, P. T., Guarraci, F. A., Olivier, J. D. A., Snoeren, E. M. S. Male rat sexual behavior: Insights from inter-copulatory intervals. Behav Processes. 190, 104458 (2021).
- Zucon Bacelar, A. C., et al. Aged and induced-premature ovarian failure mouse models affect diestrus profile and ovarian features. PLoS One. 18 (12), e0284887 (2023).
- Mouri, A., et al. Early adolescent social defeat stress exposure persistently impairs social behaviors and neurogenesis. Neuropharmacology. 133, 23-37 (2018).
- Hasegawa, S., et al. Dysfunction of serotonergic and dopaminergic neuronal systems in the antidepressant-resistant impairment of social behaviors induced by social defeat stress exposure as early adolescents. Int J Neuropsychopharmacol. 21 (9), 837-846 (2018).
- Pantoja-Urbán, A. H., et al. Gains and losses: resilience to social defeat stress in adolescent female mice. Biol Psychiatry. 95 (1), 37-47 (2023).
- Demaestri, C., et al. Resource scarcity but not maternal separation provokes unpredictable maternal care sequences in mice and both upregulate Crh-associated gene expression in the amygdala. Neurobiol Stress. 20, 100484 (2022).
- Walker, C. -. D., et al. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress. 20 (5), 421-448 (2017).
- Pardo, G. V. E., Alfaro Saca, E. E., Becerra Flores, C. T., Delgado Casós, W. F., Pacheco-Otalora, L. F. Limited bedding nesting paradigm alters maternal behavior and pup’s early developmental milestones but did not induce anxiety- or depressive-like behavior in two different inbred mice. Dev Psychobiol. 65 (1), e22357 (2023).
- Raineki, C., Cortés, M. R., Belnoue, L., Sullivan, R. M. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 32 (22), 7758-7765 (2012).
- Rincón-Cortés, M., Sullivan, R. M. Emergence of social behavior deficit, blunted corticolimbic activity and adult depression-like behavior in a rodent model of maternal maltreatment. Transl Psychiatry. 6 (10), e930 (2016).
- Raineki, C., et al. Paradoxical neurobehavioral rescue by memories of early-life abuse: the safety signal value of odors learned during abusive attachment. Neuropsychopharmacology. 40 (4), 906-914 (2015).
- Niwa, M., Matsumoto, Y., Mouri, A., Ozaki, N., Nabeshima, T. Vulnerability in early life to changes in the rearing environment plays a crucial role in the aetiopathology of psychiatric disorders. Int J Neuropsychopharmacol. 14 (4), 459-477 (2011).
- Franklin, T. B., Linder, N., Russig, H., Thöny, B., Mansuy, I. M. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One. 6 (7), e21842 (2011).
- Ronquillo, J., et al. Nature and nurture: Comparing mouse behavior in classic versus revised anxiety-like and social behavioral assays in genetically or environmentally defined groups. Genes Brain Behav. 22 (6), e12869 (2023).
Explore More Articles
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved