Zum Anzeigen dieser Inhalte ist ein JoVE-Abonnement erforderlich. Melden Sie sich an oder starten Sie Ihre kostenlose Testversion.
Measuring Brain Glutamate Levels Under Anesthesia in Neonatal Piglets Using a Microelectrode Array
In diesem Artikel
Overview
This video demonstrates the measurement of glutamate levels in the brain of an anesthetized neonatal piglet using a microelectrode array (MEA). The procedure involves creating a craniotomy window in the pig skull and inserting the MEA into the brain region of interest. The glutamate-oxidase-coated recording sites of the MEA convert extracellular brain glutamate to hydrogen peroxide, which generates a measurable current. This current is measured and normalized against background noise to determine brain glutamate levels under anesthesia.
Protokoll
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Piglets and Piglet Handling
- Utilize male and female piglets in a systematic and randomized manner to eliminate any potential sex-based confounders in accordance with ARRIVE guidelines.
NOTE: Since the period of maximal brain growth is within 3-5 days of piglet birth, experimentation is done solely with piglets 3-5 days old. - Ensure piglets arrive in the vivarium at least 24 h before experimentation to allow acclimation to the environment.
NOTE: Trained veterinary staff provides routine animal care. The piglets are kept in individually temperature-maintained, continuously monitored cages and receive a nutritionally complete milk replacer ad libitum to prevent hypoglycemia. The piglets are also kept without milk replacement (nil per os), for at least 3 h prior to anesthesia and are supplied with blankets and toys to ensure normal levels of stimulation. If possible, keep multiple piglets in the same cage to allow socialization.
2. Development and Customization of Microelectrode Arrays (MEAs) for Anesthesia-induced Neurotoxicity (AIN) Studies in a Piglet Model
NOTE: This technology uses enzyme-based MEAs that are pre-coated with enzyme and electroplated with m-phenylenediamine dihydrochloride (mPD). The electrodes were custom designed with a 40-mm rigid shaft and manufactured for use in piglets (Figure 1).
- Please see the detailed description of MEA preparation and calibration (Figure 2).
3. Anesthesia and Use of Custom Stereotaxic Apparatus for the Piglet
- Anesthetize animals using an anesthesia workstation with an appropriate ventilator and monitoring devices, and monitor physiologic parameters such as pulse oximetry, non-invasive blood pressure, electrocardiography, and temperature throughout the entirety of the experiment as previously described. Intubate and ventilate the piglets and administer sevoflurane anesthesia at 1 minimum alveolar concentration (MAC) (approximately 2.5-3%) for 3.5 h. Ensure that trained laboratory staff members are present for these experiments. Fur overlying the surgical area is removed using electric clippers prior to preparation of the skin.
NOTE: The concentration and duration of anesthetic used allows the experiment to simulate the time-length of actual anesthesia exposure during a surgical procedure. Additionally, sevoflurane is the most commonly utilized general anesthetic in the pediatric population, making the investigation surrounding its safety of utmost importance. - Before placement into the stereotaxic frame, start a rocuronium loading dose of 2.5 mg/kg and an infusion of 1.5 mg/kg/h to prevent animal movement while secured in the frame.
- Place the piglet in the piglet-specific stereotaxic frame once an adequate depth of anesthesia is confirmed by toe-pinch.
- Provide adequate padding within the stereotaxic frame by placing the piglet on a forced- air warming pad with additional padding (e.g., fluidized positioner) to prevent pressure ulcers.
- Place the teeth of the upper mandible over the tooth bar (Figure 3).
NOTE: The tooth bar should be at a sufficient level to hold the skull very firmly in place. - Fix and tighten the penetrating ear bars within the ears, taking care to ensure that the piglet is in the midline position. Position the pointed tips of the lateral holds within the ear canal and insert the ear bars firmly enough to hear the "pop" sound associated with the penetration of the tympanic membrane. Firmly attach the ear bars to the skull and insert to equal depth on each side in order to prevent movement of the skull during the experiment (Figure 4, Panel A).
NOTE: It is vitally important to keep the piglet warm (~ 37.8-38.6 °C) and continuously monitor the temperature during the entire procedure for maintenance of normothermia. This can be accomplished via a blanket and/or a heat lamp. Be sure to place the heat lamp at an appropriate distance to avoid burning of the animal's skin.
- Create a 4-6 cm midline incision along the skull using caution to avoid scoring the skull with the scalpel. Once the incision is made, use gentle retraction and blunt dissection to elevate the scalp from the skull. Gently scrub the skull with a gauze pad to remove any connective tissue and expose the suture lines (Figure 4, Panel B).
NOTE: It is not necessary to maintain sterility during non-survival experiments. However, sterility must be maintained during survival experiments. - Further reflect the scalp, if necessary, to expose the area of interest and determine the intended location for the craniotomy (Figure 5, Panel A). Use a surgical drill to create a craniotomy window of approximately 0.25 cm2 (may be larger or smaller depending on experimental goals) overlying the structure of interest, using caution not to injure the dura or the underlying brain (Figure 5, Panel B). Use fine surgical scissors to excise the dura overlying the brain tissue (Figure 5, Panel C).
- Position the electrode as vertically as possible over the bregma by securing the metal arm of the headstage to the micromanipulator of the piglet stereotax. Lower the electrode as much as possible without touching the surface of the skull. Record the coordinates of the bregma (Figure 6, Panel A).
- Determine the anterior-posterior and medial-lateral coordinates as well as the depth of the structure of interest as they relate to the bregma. Determine the stereotaxic coordinates using a species and age-appropriate stereotaxic atlas. In this case, use a stereotaxic atlas developed specifically for piglets.
- Reposition the electrode so that it has the proper anterior-posterior and medial-lateral location, ensuring that both the microelectrode and the apparatus are perpendicular to the surface. Place the pseudo-reference electrode under the scalp, ensuring contact with the animal (Figure 6, Panel B).
- Slowly and gently, lower the electrode to the appropriate depth into the brain using the stereotaxic arm. For the final 2 mm, use a hydraulic microdrive to further drive the electrode to the exact location (Figure 6, Panel C).
NOTE: The electrode position should overlie the craniotomy window. The electrode depth will vary depending on the brain structure of interest. It is not necessary to close the incision upon completion of data collection in non-survival experiments.
4. Measurement of Extracellular Glutamate Under Sevoflurane Anesthesia
- Ensure the piglet is under continuous physiologic monitoring throughout the entirety of this procedure. Piglets are anesthetized inhalationally (via face cone) in preparation for the procedure.
- After implantation of the MEA, wait 30 min to allow the electrode to reach baseline to ensure that correct measurement will be obtained for 3 h (Figure 7).
- 0.25% bupivacaine (1 mL/kg) is administered subcutaneously at the site of the surgery for postoperative pain management. In addition, sustained-release buprenorphine is given subcutaneously q72h as needed.
Ergebnisse
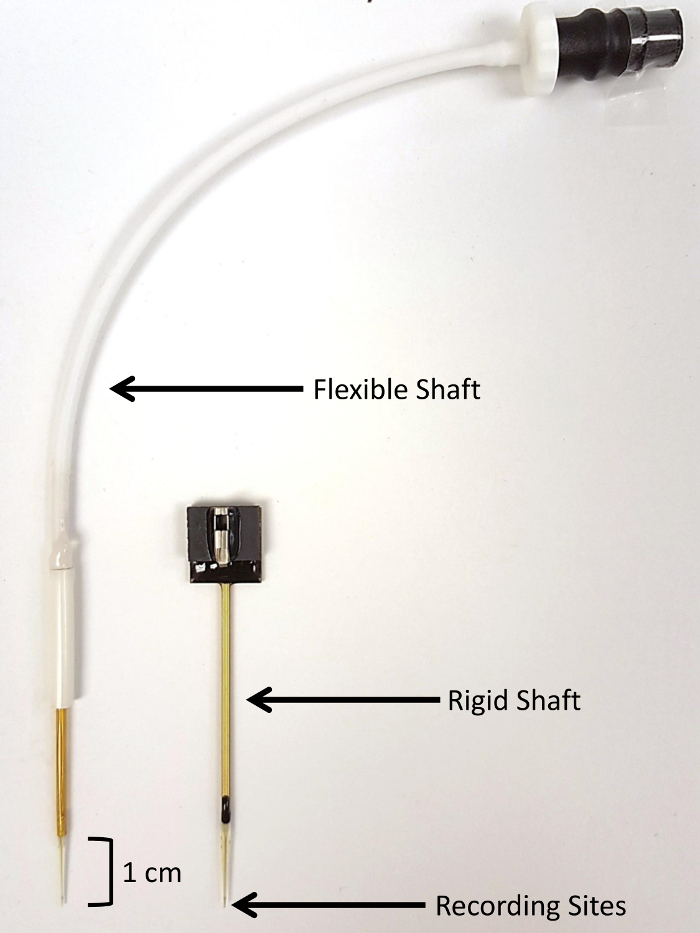
Figure 1: Visual comparison of SG-2 microelectrode array types. SG-2 arrays contain two glutamate-sensitive sites and two glutamate-insensitive sentinel sites (150 µm x 20 µm per site). (A) A flexible-shaft microelectrode array is shown on the left. The rigid-shaft microelectrode array was custom-designed for use in piglets and permits deeper implantation in large anima...
Offenlegungen
Materialien
| Name | Company | Catalog Number | Comments |
| Advance Liqui-Wean Pig Milk Replacer | PBS Animal Health | ||
| Piglet Anesthesia Face-Cone Mask | VetEquip | ||
| Integra SL Anesthesia Workstation | DRE Veterinary | This anesthesia workstation is chosen to best mimic the clinical monitoring experienced by pediatric patients in the operating room. Any anesthesia machine can be used as long as it allows for sufficient physiologic monitoring and intervention. | |
| Sevoflurane | Ultane | 0074-4456-04 | |
| Rocuronium Bromide Injection | Hospira | 0409-9558-05 | |
| Medfusion 4000 IV Infusion | Smiths Medical | ||
| Model 1530 Heavy-Duty Research Model Stereotax | Kopf | custom made | |
| Model 1541 Piglet Adaptor | Kopf | custom made | |
| Infrared Spot Lamp | Amazon | B000HHQ94C | |
| Bair Hugger Torso Blanket | 3M | 540 | |
| Bair Hugger | 3M | 750 | |
| Sterile Alcohol Prep Pad | Fisherbrand | 22-363-750 | |
| Carbon Steel Rib-Back Surgical Blade | Bard-Parker | #10 | |
| Scalpel Handel | Havel's | HAN-G4 | |
| Surgical Scissors | World Precision Instruments | 504615 | |
| Mosquito Forceps | Sklar Surgical Instruments | 17-1225 | |
| Gauze Pads | Fisherbrand | 22-246-069 | |
| Adson Tissue Forceps | Teleflex | 181223 | |
| Dremel 111 Engraving Cutter | Amazon | Dremel 111 | |
| Microelectrode Array | Center for Microelectrdoe Technology, University of Kentucky | S2 4Ch MEA; custom made | |
| Headstage | Quanteon | 2pA/mV | |
| Wire, silver, PFA, .008" Bare, .0110" coated | A-M Systems | 786500 | |
| Fine Micromanipulator | Narishige Scientific Instrument Lab | MO-8 |
Referenzen
This article has been published
Video Coming Soon
Source: Geyer, E. D., et al. Adaptation of Microelectrode Array Technology for the Study of Anesthesia-induced Neurotoxicity in the Intact Piglet Brain. J. Vis. Exp. (2018).
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten