2.1 : Réactions chimiques
Une équation chimique équilibrée fournit les informations sur les formules chimiques des réactifs et des produits impliqués dans le changement chimique. La stœchiométrie d'une réaction permet de prédire combien de réactif est nécessaire pour produire la quantité désirée de produit, ou dans certains cas, quelle quantité de produit sera formée à partir d'une quantité spécifique de réactif.
Les quantités relatives de réactifs et de produits représentées dans une équation chimique équilibrée sont souvent appelées quantités stœchiométriques. Cependant, en réalité, les réactifs ne sont pas toujours présents dans les quantités stœchiométriques indiquées par l'équation équilibrée.
Dans une réaction chimique, le réactif qui est consommé en premier, et qui limite la quantité de produit formé, est le réactif limitant, tandis que l'autre substance devient le réactif en excès. Un excès d'un ou plusieurs réactifs est souvent utilisé pour assurer la conversion complète de l'autre réactif en produit.
Considérez la réaction de formation de l'eau représentée par l'équation :
La stœchiométrie indique que deux moles d'hydrogène et une mole d'oxygène réagissent pour produire deux moles d'eau ; c'est-à-dire que l'hydrogène et l'oxygène se combinent dans un rapport de 2:1.
Imaginez si 5 moles d'hydrogène et 2 moles d'oxygène sont présentes. Le rapport des réactifs est maintenant de 5:2 (ou 2,5:1), ce qui est supérieur au rapport stœchiométrique de 2:1. L'hydrogène est donc présent en excès, et l'oxygène est le réactif limitant. La réaction de tout l'oxygène fourni (2 mol) consommera 4 mol des 5 mol d'hydrogène fournis, laissant 1 mol d'hydrogène non réagi. Le calcul des quantités molaires de chaque réactif fourni et leur comparaison avec les quantités stœchiométriques représentées dans l'équation chimique équilibrée est une façon d'identifier les réactifs limitants et en excès.
Le taux de réaction est le changement dans la quantité d'un réactif ou d'un produit par unité de temps. Les taux de réaction sont donc déterminés en mesurant la dépendance temporelle d'une propriété qui peut être reliée aux quantités de réactifs ou de produits.
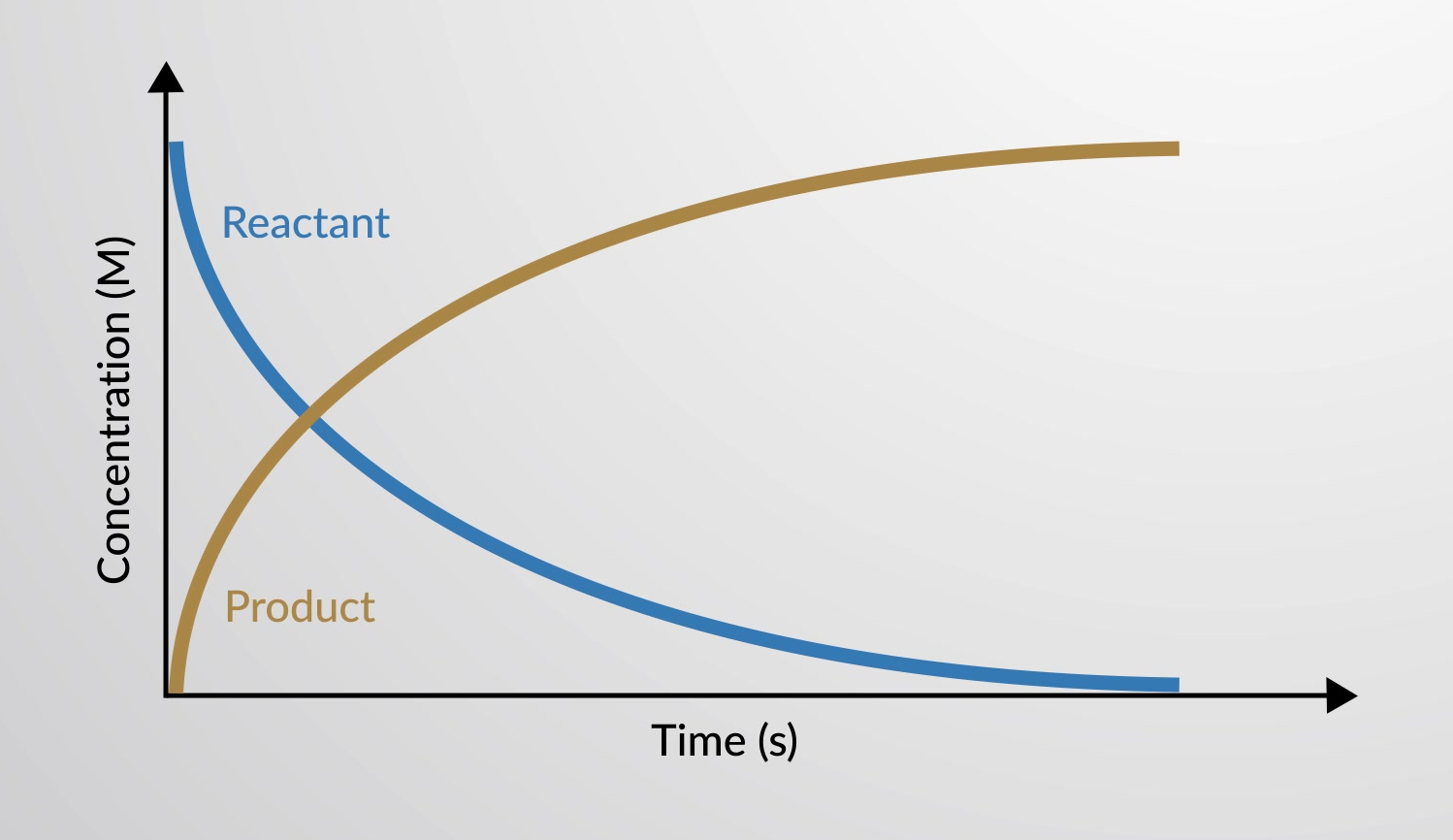
Le taux d'une réaction chimique peut être représenté dans un graphique comme la variance dans les concentrations des réactifs et des produits en fonction du temps.
Une réaction chimique réversible représente un processus chimique qui se déroule à la fois dans les directions avant (de gauche à droite) et arrière (de droite à gauche). L'état d'une réaction réversible est facilement évalué en évaluant son quotient de réaction (Q). Pour une réaction réversible décrite par
le quotient de réaction est dérivé directement de la stœchiométrie de l'équation équilibrée comme
où le subscript c indique l'utilisation de concentrations molaires dans l'expression.
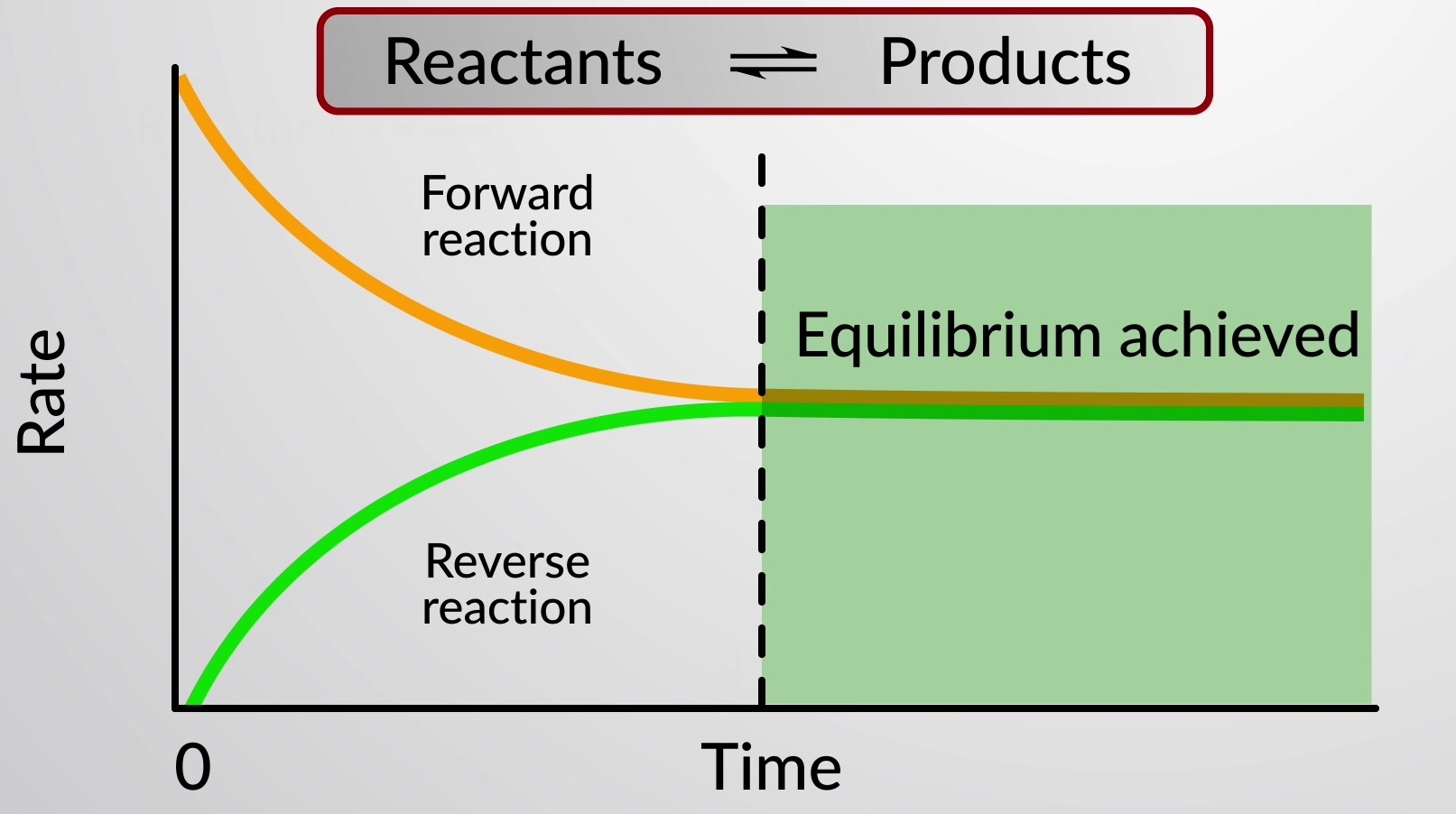
Lorsque les taux des réactions avant et arrière sont égaux, les concentrations des espèces de réactifs et de produits restent constantes dans le temps et le système est à l'équilibre. Une double flèche spéciale est utilisée pour souligner la nature réversible de la réaction.
Ce texte est adapté de OpenStax Chemistry 2e, Section 4.3: Reaction Stoichiometry; Section 4.4: Reaction Yield; Section 12.1: Chemical Reaction Rates; Section 13.1 Chemical Equilibria, Section 13.2 Equilibrium Constants.
Du chapitre 2:

Now Playing
2.1 : Réactions chimiques
Thermodynamique et cinétique chimique
9.8K Vues

2.2 : Enthalpie et chaleur de réaction
Thermodynamique et cinétique chimique
8.3K Vues

2.3 : Énergétique de la formation de la solution
Thermodynamique et cinétique chimique
6.7K Vues

2.4 : Entropie et solvatation
Thermodynamique et cinétique chimique
7.0K Vues

2.5 : Enthalpie libre et favorabilité thermodynamique
Thermodynamique et cinétique chimique
6.7K Vues

2.6 : Équilibres chimiques et équilibre de solubilité
Thermodynamique et cinétique chimique
4.1K Vues

2.7 : Loi de vitesse et ordre de réaction
Thermodynamique et cinétique chimique
9.3K Vues

2.8 : Effet du changement de température sur la vitesse de réaction
Thermodynamique et cinétique chimique
4.0K Vues

2.9 : Réactions en plusieurs étapes
Thermodynamique et cinétique chimique
7.2K Vues

2.10 : Énergie de dissociation de liaison et énergie d'activation
Thermodynamique et cinétique chimique
8.7K Vues

2.11 : Diagrammes d'énergie, états de transition et intermédiaires
Thermodynamique et cinétique chimique
16.1K Vues

2.12 : Prédire les résultats de la réaction
Thermodynamique et cinétique chimique
8.2K Vues