Un abonnement à JoVE est nécessaire pour voir ce contenu. Connectez-vous ou commencez votre essai gratuit.
A Lactate Dehydrogenase Release Assay to Detect Cryptococcus neoformans Infection in Macrophages
Dans cet article
Overview
In this video, we demonstrate a cytotoxicity assay to assess Cryptococcus neoformans, a pathogenic fungus, infection in macrophages. The cytotoxicity is determined by the release of cytoplasmic lactate dehydrogenase enzyme due to macrophage lysis caused by Cryptococcus neoformans infection.
Protocole
1. Verification of infection
NOTE: Using a cytotoxicity assay, infection proficiency can be measured. The following protocol will highlight the application of a cytotoxicity product to measure LDH (lactate dehydrogenase) release. Other cytotoxicity products can also be used
- Preparation of C. neoformans infection of macrophage cells
- Preparation of fungal cells
- Using a glycerol stock, streak wildtype C. neoformans strain (H99) onto Yeast-extract peptone dextrose (YPD) agar plate to isolate single colonies.
- Incubate overnight for 16 h at 37 °C in a static incubator.
- Select a single colony of wildtype C. neoformans strain and culture in 5 mL of YPD broth in a loosely capped 10 mL test tube. Perform in quadruplicate.
- Incubate overnight for 16 h at 37 °C in a shaking incubator at 200 rpm.
- The following day subcultures each overnight culture in a 1:100 dilution into 3 mL YPD broth.
- Measure Optical density (OD600nm) values of fungal culture to determine the mid-log phase. Depending on the spectrophotometer, C. neoformans wild-type strain reaches the mid-log phase commonly following 6 to 8 h incubation in YPD with general OD600nm values ranging from 1.0 to 1.5.
- Once cells reach the mid-log phase, take a 10 µL aliquot of fungal cell suspension into a clean 1.5 mL microcentrifuge tube and dilute 1:100 in sterile 1x phosphate-buffered saline (PBS). Count the number of cells using a hemocytometer.
- Collect and centrifuge cells at 1,500 x g for 10 min, gently wash the pellet with sterile room-temperature PBS, and repeat for a total of three washes.
- Resuspend cells in an antibiotic-free cell culture medium to achieve a concentration of 1.2 x 108 cells/mL.
- Preparation of macrophage cells
- Visualize each well in the 6-well plate to ensure that cells have reached 70-80% confluence. Alternatively, cells can be measured to achieve approx. 1.2 x 106 macrophage cells per well.
- Warm antibiotic-supplemented medium to 37 °C prior to cell culture work. Ensure the work environment is sterilized prior to cell culture work. Visualize cells using a light microscope to ensure cells are adherent and healthy.
- Remove the cell culture medium from the dish either with a serological pipette or a vacuum aspirator.
- Gently add 5-7 mL of sterile room-temperature PBS (phosphate-buffered saline) to the 60 x 15 mm dish.
- Gently tilt the dish to wash adhered cells.
- Co-culture of C. neoformans and macrophage cells
- Add 1 mL of the resuspended C. neoformans cells to 4 wells containing macrophage cells.
NOTE: The number of plates required will need to be calculated prior to beginning the experiment. Add 1 mL of antibiotic-free medium to empty wells. - Allow cells to incubate at 37 °C with 5% CO2 for 3 h.
- Remove the cell culture medium from the plate either with a serological pipette or a vacuum aspirator.
- Gently add 1 mL of sterile room-temperature PBS.
- Gently tilt the plate to wash non-attached or non-phagocytosed extracellular C. neoformans cells.
- Add 1 mL of antibiotic-free medium to each well in the 6-well plate.
- Incubate cells at 37 °C with 5% CO2.
- Add 1 mL of the resuspended C. neoformans cells to 4 wells containing macrophage cells.
- At selected time points (e.g., 1, 3, 6, 12, and 24 hours) collect supernatant for measurement of LDH release according to the manufacturer's instructions.
- At the same time points, uninfected macrophage cells will be lysed to a determined value for maximum cytotoxicity.
- Calculate cytotoxicity as follows:
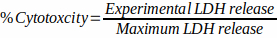
- Preparation of fungal cells
Déclarations de divulgation
matériels
| Name | Company | Catalog Number | Comments |
| 100 mM Tris-HCl, pH 8.5 | Fisher Scientific | BP152-1 | Maintain at 4°C |
| 60 x 15 mm Dish, Nunclon Delta | ThermoFisher Scientific | 174888 | |
| 6-well cell culture plate | ThermoFisher Scientific | 140675 | |
| Conical falcon tubes (15 mL) | Fisher Scientific | 05-539-12 | |
| Countess II Automated Cell Counter | ThermoFisher Scientific | AMQAX1000 | Not essential, haemocytometer can be used as an alternative. |
| CytoTox 96 Non-Radioactive Cytotoxicity Assay | Promega | G1780 | |
| DMEM, high glucose, GlutaMAX Supplement | ThermoFisher Scientific | 10566016 | |
| Fetal Bovine Serum (FBS) | ThermoFisher Scientific | 12483020 | Heat inactivate by incubating at 60°C for 30 minutes. Prepare 50 ml aliquots and flash freeze. Thaw prior to media preparation |
| Hemocytometer | VWR | 15170-208 | |
| HEPES | Sigma Aldrich | H3375 | Prepare 40 mM HEPES/8 M Urea in bulk stock solution, flash frozen, store at -20°C until use. Discard after each use (do not freeze-thaw repeatedly). |
| L-glutamine | ThermoFisher Scientific | 25030081 | Can be aliquot and frozen for storage. Thaw prior to media preparation. |
| Microcentrifuge | Eppendorf | 13864457 | |
| Penicillin : Streptomycin 10k/10k | VWR | CA12001-692 | Can be aliquot and frozen for storage. Thaw prior to media preparation. |
| Phosphate-Buffered Saline | VWR | CA12001-676 | Purchase not required. PBS can also be prepared but sterile filteration must be performed before use. |
| Pipette, Disposable Serological (10 mL) | Fisher Scientific | 13-678-11E | |
| Pipette, Disposable Serological (25 mL) Basix | Fisher Scientific | 14955235 | |
| Trypsin/Lys-C protease mix, MS grade | Pierce | A40007 | Maintain at -20 °C. |
| Urea | Sigma Aldrich | U1250-1KG | Prepare 40 mM HEPES/8 M Urea in bulk stock solution, flash frozen, store at -20 °C until use. Discard after each use (do not freeze-thaw repeatedly). |
| Yeast-extract peptone dextrose broth | BD Difco | BM20 |
This article has been published
Video Coming Soon
Source: Ball, B., et al. Label-Free Quantitative Proteomics Workflow for Discovery-Driven Host-Pathogen Interactions. J. Vis. Exp. (2020)