The pH of a solution containing an acid can be determined using its acid dissociation constant and its initial concentration. If a solution contains two different acids, then its pH can be determined using one of several methods depending upon the relative strength of the acids and their dissociation constants.
A Mixture of a Strong Acid and a Weak Acid
In a mixture of a strong acid and a weak acid, the strong acid dissociates completely and becomes a source of almost all the hydronium ions present in the solution. In contrast, the weak acid shows partial dissociation and produces a negligible concentration of hydronium ions. The high concentration of hydronium ions produced by the strong acid further reduces the dissociation of the weak acid. This happens because, according to Le Chatelier’s principle – “When a chemical system at equilibrium is disturbed, the system shifts in a direction that minimizes the disturbance.” The excess hydronium ions produced by the strong acid disturbs the equilibrium, and thus the reaction will move in the reverse direction until the equilibrium is established. This leads to a decrease in the dissociation of the weak acid. Because of this decrease, a pH of a mixture of a strong and weak acid can be calculated from the concentration of the strong acid only. For example, the pH of a mixture with an equal concentration of hydrochloric acid (HCl), a strong acid, and formic acid (HCHO2), a weak acid, can be determined from the concentration of HCl only. If the concentration of the HCl in the mixture is 0.0020 M, its pH can be calculated as follows.
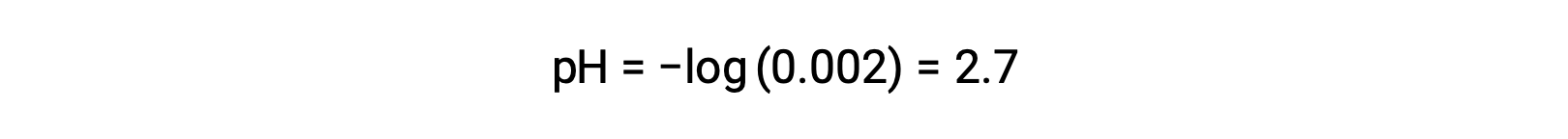
Here, the concentration of hydronium ions produced by HCHO2 and the autoionization of water is negligible and thus can be ignored.
A Mixture of Two Weak Acids with Different Dissociation Constants
In a mixture of two weak acids, the pH of a mixture will be determined by the stronger acid if its dissociation constant is significantly higher than the weaker acid. For example, in a mixture with an equal concentration of nitrous acid (HNO2) and hypochlorous acid (HClO), the HNO2 will be the main determinant of the pH of the mixture as its Ka (4.6 × 10−4) is approximately 10,000 times higher than the Ka (2.9 × 10−8) of HClO. According to Le Chatelier’s principle, HClO shows decreased dissociation in the presence of HNO2.
From Chapter 15:

Now Playing
15.9 : תערובת של חומצות
Acids and Bases
19.3K Views

15.1 : ברונסטד-לאורי חומצות ובסיסים
Acids and Bases
88.1K Views

15.2 : חוזקי חומצה/בסיס וקבועי פירוק
Acids and Bases
59.1K Views

15.3 : מים: חומצת ובסיס ברונסטד-לאורי
Acids and Bases
48.6K Views

15.4 : סקאלת pH
Acids and Bases
66.8K Views

15.5 : חוזקים יחסיים של זוגות חומצה-בסיס מצומדים
Acids and Bases
44.4K Views

15.6 : תמיסות חומצה ובסיס חזקים
Acids and Bases
30.6K Views

15.7 : תמיסות חומצה חלשה
Acids and Bases
36.9K Views

15.8 : תמיסות בסיס חלש
Acids and Bases
21.9K Views

15.10 : יונים כחומצות ובסיסים
Acids and Bases
22.7K Views

15.11 : קביעת הpH של תמיסות מלח
Acids and Bases
42.7K Views

15.12 : חומצות רב פרוטיות
Acids and Bases
28.3K Views

15.13 : חוזק חומצה ומבנה מולקולרי
Acids and Bases
30.2K Views

15.14 : חומצות ובסיסי לואיס
Acids and Bases
42.4K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved