Isolamento simultaneo dei principali tipi di cellule residenti nel sistema nervoso centrale da topi adulti con encefalomielite autoimmune
* Questi autori hanno contribuito in egual misura
In questo articolo
Riepilogo
Ad oggi, i protocolli per l'isolamento simultaneo di tutti i principali tipi di cellule residenti nel sistema nervoso centrale dallo stesso topo sono una richiesta insoddisfatta. Il protocollo mostra una procedura applicabile nei topi con encefalomielite autoimmune naïve e sperimentale per studiare reti cellulari complesse durante la neuroinfiammazione e contemporaneamente ridurre il numero di topi richiesti.
Abstract
L'encefalomielite autoimmune sperimentale (EAE) è il modello murino più comune per la sclerosi multipla (SM) ed è spesso utilizzato per chiarire ulteriormente l'eziologia ancora sconosciuta della SM al fine di sviluppare nuove strategie di trattamento. Il modello EAE del peptide glicoproteico oligodendrocitario della mielina 35-55 (MOG35-55) riproduce un decorso autolimitante della malattia monofasica con paralisi ascendente entro 10 giorni dall'immunizzazione. I topi vengono esaminati quotidianamente utilizzando un sistema di punteggio clinico. La SM è guidata da diversi meccanismi patogenetici con uno specifico pattern temporale, quindi lo studio del ruolo dei tipi di cellule residenti nel sistema nervoso centrale (SNC) durante la progressione della malattia è di grande interesse. La caratteristica unica di questo protocollo è l'isolamento simultaneo di tutti i principali tipi di cellule residenti nel SNC (microglia, oligodendrociti, astrociti e neuroni) applicabili nell'EAE adulto e nei topi sani. La dissociazione del cervello e del midollo spinale dai topi adulti è seguita dallo smistamento cellulare attivato magneticamente (MACS) per isolare microglia, oligodendrociti, astrociti e neuroni. La citometria a flusso è stata utilizzata per eseguire analisi di qualità delle sospensioni monocellulari purificate, confermando la vitalità dopo l'isolamento cellulare e indicando la purezza di ciascun tipo di cellula di circa il 90%. In conclusione, questo protocollo offre un modo preciso e completo per analizzare reti cellulari complesse in topi sani e EAE. Inoltre, il numero di topi richiesti può essere sostanzialmente ridotto poiché tutti e quattro i tipi di cellule sono isolati dagli stessi topi.
Introduzione
La sclerosi multipla (SM) è una malattia infiammatoria cronica autoimmune del sistema nervoso centrale (SNC) caratterizzata da demielinizzazione, danno assonale, gliosi e neurodegenerazione. Nonostante i numerosi approcci di ricerca in questo campo, la fisiopatologia della SM non è ancora pienamente compresa 1,2,3,4. Il modello animale più comune per lo studio della SM è l'encefalomielite autoimmune sperimentale (EAE) indotta dal peptide glicoproteico oligodendrocitario della mielina 35-55 (MOG35-55), che condivide molte delle sue caratteristiche cliniche e fisiopatologiche 5,6,7,8,9 . Si basa sulla risposta del sistema immunitario contro gli antigeni specifici del SNC che portano all'infiammazione, alla demielinizzazione e alla degenerazione neuroassonale. L'encefalomielite autoimmune sperimentale (EAE) è un modello adatto per lo studio delle vie neuroinfiammatorie e delle cascate di segnalazione riscontrate nella SM.
Le attuali opzioni terapeutiche per la SM sono solo parzialmente efficaci e si concentrano principalmente sulla fase infiammatoria iniziale della malattia. Tuttavia, la componente neurodegenerativa della SM sembra essere la sfida principale per gli approcci terapeutici a lungo termine. Pertanto, sono necessari protocolli di isolamento cellulare riproducibili e precisi per studiare in modo completo i meccanismi molecolari e cellulari nelle malattie autoimmuni. Anche se esistono alcuni protocolli per l'isolamento di un singolo tipo di cellula 10,11,12,13,14,15, c'è un'esigenza insoddisfatta per l'isolamento simultaneo di più popolazioni cellulari residenti nel SNC contemporaneamente. I precedenti protocolli per l'isolamento delle cellule residenti nel SNC non sono in grado di preservare la funzionalità e la purezza cellulare, con conseguente co-coltivazione con cellule vicine 16,17,18 o l'inidoneità per analisi complesse di reti intracellulari ex vivo 19,20,21,22.
Lo scopo di questo protocollo è stato quello di stabilire un metodo riproducibile e completo per l'isolamento simultaneo di sospensioni unicellulari vitali pure di tutti i principali tipi di cellule residenti nel SNC, applicabile in topi adulti sani e EAE. I diversi tipi di cellule sono stati isolati utilizzando il cervaglio cellulare attivato magneticamente (MACS)23. La separazione cellulare può essere ottenuta sia mediante selezione positiva, cioè marcatura magnetica di marcatori di superficie specifici per tipo di cellula, sia mediante selezione negativa tramite biotinilazione e svuotamento di tutte le cellule indesiderate. La citometria a flusso è stata applicata per garantire una purezza superiore al 90% e una vitalità di almeno l'80% delle sospensioni isolate a singola cellula.
In conclusione, l'obiettivo principale è stato quello di stabilire un protocollo per l'isolamento simultaneo di tutti i principali tipi di cellule residenti nel SNC come strumento versatile per lo studio delle vie neuroinfiammatorie offrendo un'analisi completa e precisa di reti cellulari complesse e cascate di segnalazione biochimica in topi sani e EAE.
Protocollo
Tutti gli esperimenti EAE sono stati indotti in topi femmina C57BL/6J all'età di 10-12 settimane e approvati dalle autorità locali (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen). Anche il rispetto della legge tedesca e dell'UE sulla protezione degli animali è stato garantito in qualsiasi momento degli esperimenti. Tutti i topi sono stati tenuti in gabbie ventilate individualmente.
NOTA: I seguenti volumi di reagenti si riferiscono a un cervello e a un midollo spinale murini adulti, che sono denominati sospensione di cellule del SNC nel seguente e pesano approssimativamente da circa 20 mg a 500 mg. Se è prevista la dissociazione di più di una sospensione cellulare del SNC, tutti i volumi e i materiali dei reagenti devono essere aumentati di conseguenza. Si raccomanda di conservare continuamente in ghiaccio la soluzione salina tamponata con fosfato di Dulbecco (D-PBS; 1x) con calcio e magnesio, integrata con 1 g/L di glucosio e 36 mg/L di piruvato di sodio) durante l'intero esperimento. Se in seguito è prevista la coltivazione delle cellule, eseguire tutte le fasi in condizioni sterili mediante l'uso di cappe. In caso contrario, nessuna delle seguenti sezioni del protocollo deve essere eseguita sotto un cofano. Conservare i tamponi sul ghiaccio. Utilizzare solo soluzioni pre-raffreddate ed evitare il vortice durante l'intero esperimento. Vedere la Figura 1 per il flusso di lavoro generale.
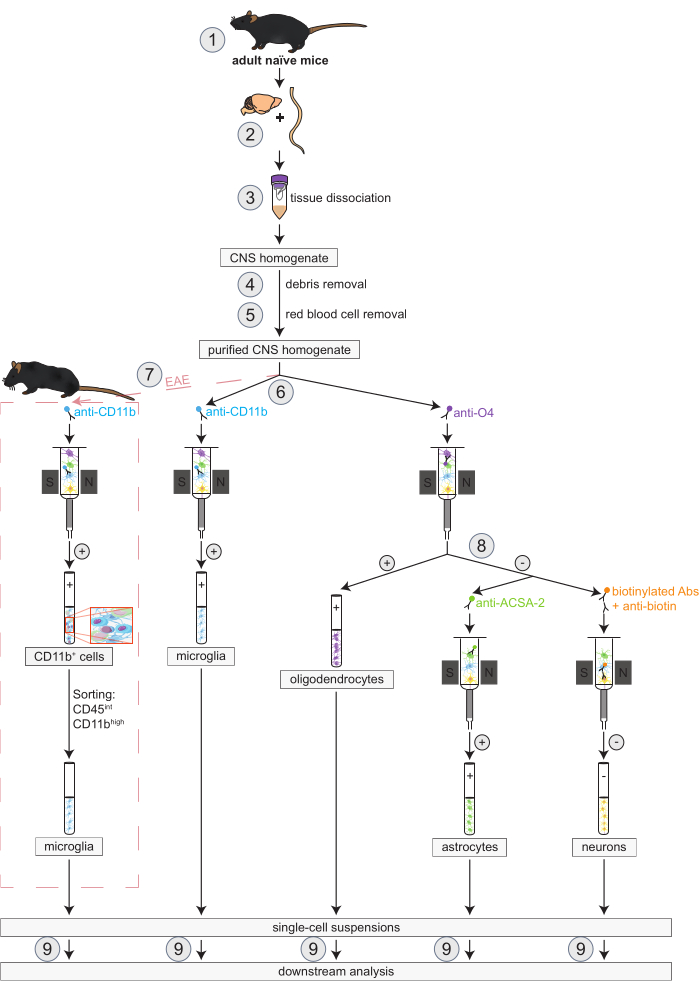
Figura 1: Flusso di lavoro per l'isolamento simultaneo di oligodendrociti, microglia, astrociti e neuroni in topi naïve e EAE. I primi passi del flusso di lavoro sono gli stessi sia per i topi naïve che per quelli EAE. Se si desidera lavorare con una replica EAE, l'induzione EAE deve essere eseguita in anticipo (1). In breve, il protocollo inizia con la dissezione (2) e la dissociazione (3) del cervello murino e del midollo spinale, seguita dalla rimozione dei detriti (4) e dei globuli rossi (5). Successivamente, la sospensione cellulare del SNC purificata risultante viene suddivisa in due frazioni per l'isolamento simultaneo di oligodendrociti e microglia tramite MACS (6). Le microglia vengono rilevate tramite microsfere anti-CD11b, mentre gli oligodendrociti vengono isolati utilizzando microsfere anti-O4 (selezioni positive). Dal flusso negativo degli oligodendrociti (8), gli astrociti vengono isolati tramite microsfere anti-ACSA-2 (selezione positiva) e neuroni mediante marcatura con biotina e deplezione di tutte le cellule non neuronali (selezione negativa). Nei topi EAE, l'isolamento delle cellule CD11b+ è seguito dall'ordinamento cellulare attivato dalla fluorescenza delle cellule CD45intCD11balte per eliminare altre cellule immunitarie CD11b+ come macrofagi, cellule dendritiche, monociti, granulociti e cellule natural killer che sono note per partecipare ai processi di neuroinfiammazione durante il corso EAE (7)27,28,48. Dopo l'isolamento dei diversi tipi di cellule residenti nel SNC, è possibile eseguire analisi di purezza (9). Abbreviazioni: Abs = anticorpi; ACSA-2 = antigene di superficie delle cellule astrocitarie-2; CD11b = chinasi ciclina-dipendente 11B; CD45 = recettore tirosin-proteina fosfatasi C; SNC = sistema nervoso centrale; EAE = encefalomielite autoimmune sperimentale; MACS = selezione cellulare ad attivazione magnetica; O4 = marcatore oligodendrocitario O4. Questa cifra è stata modificata da49. Fare clic qui per visualizzare una versione più grande di questa figura.
1. Induzione di EAE attivi
- Preparazione dei reagenti
- Per la separazione cellulare: preparare il tampone PB e conservarlo a 2-8 °C per un massimo di 1 settimana. Per preparare la soluzione madre, aggiungere 475 mL di 1x PBS senza integratori (pH 7,2) + 25 mL di albumina sierica bovina allo 0,5% (BSA). Utilizzare una diluizione 1:20 preparata in BSA.
- Per la citometria a flusso e lo smistamento cellulare attivato dalla fluorescenza (FACS): preparare il tampone FACS, PBS con siero fetale di vitello (FCS) al 2% e 2 mM di EDTA e conservarlo a 2-8 °C. Per preparare, aggiungere 500 mL di 1x PBS senza supplementi e 10 mL di FCS + 2 mL di EDTA (da 0,5 M di EDTA)
- Eseguire l'immunizzazione secondo il protocollo di Bittner et al. 5. In breve, indurre EAE mediante iniezione sottocutanea di un'emulsione contenente 200 μg di peptide MOG35-55 e 200 μL di adiuvante di Freud completo di cui 200 μg di Mycobacterium tuberculosis.
- Anestetizzare il topo con isoflurano al 2% utilizzando una camera di anestesia con un vaporizzatore di isoflurano. Usa un unguento veterinario sugli occhi dell'animale per prevenire la secchezza durante l'anestesia.
- Dopo 2 ore, iniettare un'iniezione intraperitoneale di 100 ng di tossina pertosse (PTx) disciolta in 100 μL di PBS 1x secondo il protocollo di Huntemann et al.24. Ripetere l'iniezione di PTx il giorno 2 dopo l'immunizzazione.
ATTENZIONE: Osservare ogni animale fino a quando non ha ripreso conoscenza sufficiente per mantenere il decubito sternale. I topi che hanno subito le procedure di iniezione non vengono restituiti alla compagnia degli altri topi fino a quando non si sono completamente ripresi. Per Mycobacterium tuberculosis e PTx: evitare l'inalazione, l'ingestione e il contatto con la pelle e gli occhi. Il Mycobacterium tuberculosis è un attivatore del sistema immunitario innato. Il PTx ha molti effetti biologici. - Monitorare quotidianamente la progressione dell'EAE, eseguita da due ricercatori in cieco che monitorano il peso ed esaminano clinicamente i topi.
- A tal fine, utilizzare il seguente sistema di punteggio: grado 0 - nessun segno clinico di EAE, grado 1 - paresi parziale della coda, grado 2 - paresi completa della coda, grado 3 - debolezza moderata degli arti posteriori, grado 4 - debolezza completa degli arti posteriori e andatura atassica, grado 5 - paraparesi lieve, paraparesi di grado 6, paraparesi di grado 7, tetraparesi di grado 8, tetraparesi di grado 9, e morte di grado 10.
- Utilizzare i seguenti criteri di esclusione per un'ulteriore partecipazione al punteggio clinico dell'esperimento > 7 o una perdita di peso superiore al 20% del peso corporeo iniziale.
- Per la dissezione del cervello e del midollo spinale, sopprimere i topi EAE il giorno 16 dopo l'induzione EAE che rappresenta il massimo della malattia.
2. Preparazione del tessuto nervoso centrale (durata: circa 10 minuti per topi)
- Dopo aver sacrificato i topi con anidride carbonica, inizia con la perfusione transcardica di ciascun topo con 20 ml di PBS 1x. Ripetere nuovamente la perfusione con 20 mL di PBS 1x.
- Posizionare il mouse in posizione supina e fissare gli arti con le cannule. Applicare etanolo al 75% sulla parte anteriore del corpo dell'animale. A quel punto non sono necessarie ulteriori misure di sterilità.
- Apri l'addome e il torace facendo una sezione longitudinale attraverso la pelle e la fascia con l'aiuto di una forbice.
- Taglia le costole lateralmente e piega il torace verso l'alto per ottenere libero accesso al cuore. Fissare il torace piegato verso l'alto con le cannule.
- Apri l'atrio destro usando le forbici. Applicare 20 ml di PBS 1x nel ventricolo sinistro con una cannula per eliminare il sangue attraverso l'atrio destro inciso.
- Esporre il cranio tagliando la pelle sopra la testa murina attraverso una sezione longitudinale e spostare la pelle intorno alla testa usando una pinza. Incidere il cranio con l'aiuto di una forbice lungo la sutura sagittale.
- Inserire la punta di una pinza lungo la linea di incisione per aprire la calotta. Rimuovere le parti rimanenti della calotta con una pinza in modo che il cervello sia completamente esposto.
- Rimuovi il cervello con cura e posizionalo in una matrice cerebrale murina. Tagliate il cervello a fette sagittali spesse 1 mm usando una lametta da barba.
- Tagliare la colonna vertebrale con l'aiuto di una forbice appena sopra la cresta iliaca in modo che la siringa possa essere inserita nel canale spinale.
NOTA: Il modo più semplice per rimuovere il midollo spinale è risciacquarlo dal canale spinale con PBS. In caso contrario, gli archi vertebrali devono essere aperti singolarmente con le forbici e quindi il midollo spinale può essere rimosso. - Sciacquare il midollo spinale dal canale spinale dalla caudale al craniale utilizzando una siringa da 20 ml con un ago da 20 G contenente 1x PBS. Tagliare il midollo spinale in segmenti lunghi 0,5 cm con un bisturi.
- Conservare ogni sospensione di cellule del SNC costituita da cervello e midollo spinale corrispondente in una capsula di Petri separata per topo riempita con circa 3 mL di D-PBS freddo. Conservare le pietanze con ghiaccio fino a un'ulteriore lavorazione.
3. Dissociazione tissutale del SNC (Durata: circa 1-1,5 h a seconda del numero di sospensioni cellulari del SNC)
NOTA: Il tessuto neurale di topi adulti viene dissociato combinando la dissociazione meccanica con la degradazione enzimatica della matrice extracellulare. In tal modo, l'integrità strutturale rimane e la sospensione cellulare può essere utilizzata per ulteriori procedure di isolamento cellulare.
- Preparare il volume appropriato della miscela enzimatica 1 composta da 50 μL di enzima P e 1.900 μL di tampone Z per sospensione cellulare del SNC. Entrambi i reagenti appartengono al kit di dissociazione cerebrale per adulti.
- Preparare il volume appropriato della miscela enzimatica 2 costituita da 10 μL di enzima A e 20 μL di tampone Y per sospensione cellulare del SNC. Entrambi i reagenti appartengono al kit di dissociazione cerebrale per adulti.
- Trasferire 1.950 μL di miscela enzimatica 1 nella provetta C e aggiungere successivamente i pezzi di tessuto di una sospensione cellulare del SNC. Utilizzare un tubo C per mouse.
- Aggiungere 30 μL di miscela enzimatica 2 a ciascuna provetta C. Chiudere bene i tubi C e fissarli capovolti sul manicotto del dissociatore di celle con riscaldatori.
- Eseguire il programma appropriato denominato 37C_ABDK_01 (richiede 30 minuti). Osservare almeno i primi 5 minuti del programma per assicurarsi che tutti i tubi girino alla stessa velocità. È possibile che si verifichino errori durante l'esecuzione. Quindi, vai al passaggio 6.
- Negli ultimi 2 minuti del programma, posizionare un colino da 70 μm su una provetta da 50 mL per ogni sospensione di cellule del SNC dissociata. Pre-inumidire questi filtri con 2 mL di D-PBS.
- Al termine del programma, collegare le provette C dal dissociatore e inserirle in una centrifuga. Centrifugare i campioni a 300 x g e 4 °C per 1 minuto per raccogliere il campione sul fondo della provetta.
- Risospendere il campione e applicarlo al colino pre-inumidito. Aggiungere 10 mL di D-PBS freddo alla provetta C vuota e chiuderla. Agitare delicatamente e applicare la sospensione sul colino corrispondente.
- Gettare i filtri e chiudere le provette da 50 ml. Centrifugare nuovamente la sospensione cellulare a 300 x g e 4 °C per 10 min. Successivamente, aspirare l'intero surnatante con molta attenzione.
4. Rimozione dei detriti (Durata: circa 1,5-2 ore a seconda del numero di sospensioni cellulari del SNC)
NOTA: La dissociazione tissutale spesso porta a mielina e detriti cellulari che possono compromettere l'analisi a valle. Con l'aggiunta di una soluzione per la rimozione dei detriti, questi detriti possono essere rimossi in modo efficiente dalla sospensione cellulare del SNC.
- Risospendere accuratamente il pellet cellulare con 3.100 μL di D-PBS per ciascuna sospensione cellulare del SNC. Non vorticare.
- Se si lavora con più di una sospensione di cellule del SNC, raggruppare al massimo due sospensioni di cellule del SNC derivate da una condizione o da un gruppo sperimentale in una provetta da 15 mL.
- Aggiungere 900 μL della soluzione per la rimozione dei detriti dal kit di dissociazione cerebrale per adulti a una sospensione di cellule del SNC o 1.800 μL di soluzione per la rimozione dei detriti a due sospensioni di cellule del SNC raggruppate.
- Capovolgere il tubo e mescolare la sospensione. Successivamente, sovrapporlo molto delicatamente con 4 ml di D-PBS freddo. Dovrebbe essere visibile un gradiente chiaro (Figura 2A).
- Centrifugare le provette per 10 minuti a 3000 x g e 4 °C con la massima accelerazione e senza freno.
- Se la separazione avviene come previsto, si formano tre fasi (Figura 2C). Aspirare completamente le due fasi superiori (Figura 2C-1,2) ed eliminarle. È importante che non rimangano residui di mielina (Figura 2E).
NOTA: Se il gradiente non ha funzionato e le celle sono necessarie urgentemente, non aspirare le due fasi superiori. Invece, riempi la provetta da 15 mL con D-PBS freddo fino a 15 mL e capovolgi più volte. Centrifugare nuovamente a 1000 x g per 10 minuti a 4 °C con la massima accelerazione e senza freno. Aspirare il surnatante e ripetere i passaggi 4.1-4.4. - Riempire la provetta con D-PBS freddo fino a 14 mL e chiuderla. Capovolgere con forza il tubo sul banco di lavoro fino a quando il pellet cellulare non si stacca dal fondo del tubo. Non vorticare.
- Centrifugare nuovamente il campione a 1000 x g e 4 °C per 10 min. Impostare l'accelerazione completa e il freno completo. Aspirare il surnatante con cura e completamente.
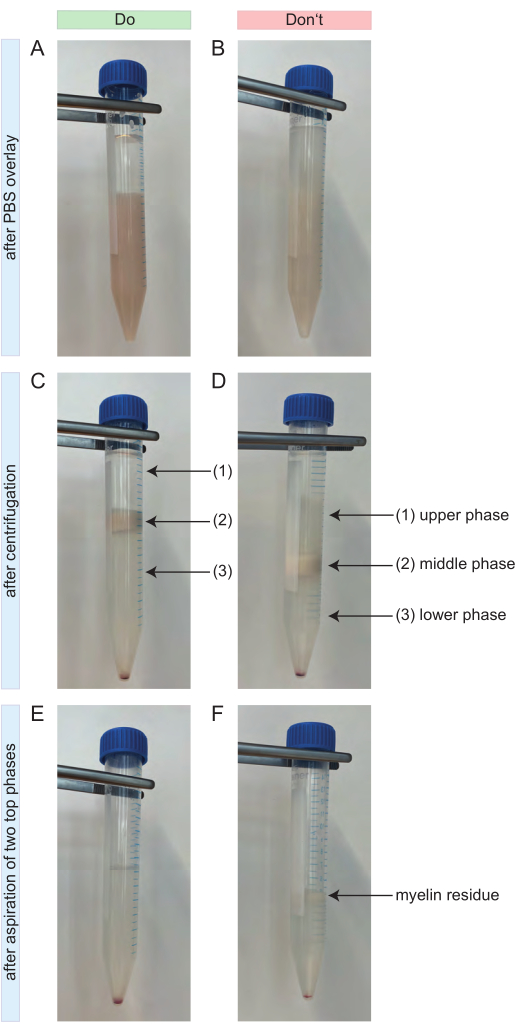
Figura 2: Cosa fare e cosa non fare durante la rimozione dei detriti. (A) Esempio positivo per il gradiente dopo la sovrapposizione con 4 mL di PBS. La fase superiore costituita da 4 mL di PBS è chiaramente distinguibile dalla fase inferiore costituita dalla sospensione cellulare del SNC con la soluzione di rimozione dei detriti. (B) Esempio negativo per il gradiente dopo la sovrapposizione con 4 mL di PBS. Il gradiente manca di una netta separazione tra il PBS e la sospensione cellulare sottostante. Un po' di PBS viene diffuso nella sospensione cellulare. (C) Esempio positivo per il gradiente dopo centrifugazione. Si possono facilmente distinguere tre fasi distinte. Non sono visibili residui di mielina nella fase superiore (1) o inferiore (3) del gradiente. La fase intermedia contiene tutta la mielina (2). Il pellet cellulare è visibile sul fondo della provetta da 15 ml. (D) Esempio negativo per il gradiente dopo centrifugazione. Non è possibile una separazione accurata tra le tre fasi. Alcuni residui di mielina sono visibili nella fase superiore (1) e inferiore (3) del gradiente. (E) Esempio positivo per il gradiente dopo aver aspirato le due fasi superiori. Il campione risultante contiene solo il pellet cellulare e un surnatante trasparente sopra. Non vengono lasciati residui di mielina. (F) Esempio negativo per il gradiente dopo aver aspirato le due fasi superiori. Il campione contiene ancora alcuni residui di mielina (freccia nera). Abbreviazioni: SNC = sistema nervoso centrale; PBS = soluzione salina tamponata con fosfati Fare clic qui per visualizzare una versione più grande di questa figura.
5. Rimozione dei globuli rossi (durata: circa 1 ora a seconda del numero di sospensioni di cellule del SNC)
NOTA: Questo passaggio previene la successiva contaminazione da parte dei globuli rossi e garantisce una lisi ottimale degli eritrociti con un effetto minimo sugli altri tipi di cellule isolate dal tessuto del SNC. I seguenti volumi sono indicati per sospensioni cellulari derivate da 100 mg a 1 g di tessuto neuronale corrispondente a due cervelli di topi adulti e midollo spinale. Se si lavora con più di due sospensioni cellulari del SNC, aumentare di conseguenza tutti i reagenti e i volumi totali.
- Iniziare con la preparazione della soluzione per la rimozione dei globuli rossi (RBCRS): per due sospensioni di cellule del SNC raggruppate. Diluire 100 μL di soluzione madre per la rimozione dei globuli rossi (10x) dal kit di dissociazione cerebrale per adulti in 900 μL di ddH2O per ottenere una diluizione finale di 1:10.
- Conservare l'RBCRS a 2-8 °C fino al momento dell'uso. Scartare i resti inutilizzati alla fine della giornata.
- Risospendere il pellet cellulare di un massimo di due sospensioni cellulari del SNC in 1 mL dell'RBCRS. Evita il vortice. Incubare la soluzione per 10 minuti a 4 °C.
- Aggiungere 10 mL di tampone PB freddo a due sospensioni cellulari in pool. Centrifugare il campione a 300 x g e 4 °C per 10 minuti e successivamente aspirare completamente il surnatante.
- Risospendere ciascun pellet cellulare da una sospensione cellulare del SNC in 80 μL di tampone PB pipettando lentamente su e giù. Di conseguenza, utilizzare 160 μL per risospendere i pellet cellulari derivati da due sospensioni cellulari del SNC.
- Quando si lavora con diverse sospensioni cellulari del SNC provenienti dalla stessa condizione sperimentale, raggruppare tutte queste sospensioni cellulari.
- Determinare il numero di cellule, ad esempio utilizzando una camera di conteggio migliorata. Le sospensioni cellulari sono state solitamente diluite 1:50 in tampone PB, seguita da un'ulteriore diluizione di 1:10 in soluzione di blu di tripano allo 0,4%.
6. Protocollo delle biglie magnetiche in topi naïve e EAE (Durata: 1 ora circa)
- Etichetta magneticamente i diversi tipi di cellule del SNC con MicroBeads specifici per il loro antigene di superficie. Quindi, posizionare la sospensione cellulare nella colonna e separare magneticamente le cellule etichettate trattenute all'interno della colonna e le celle non etichettate che scorrono attraverso.
- Dopo aver rimosso la colonna dal campo magnetico, svuotare le cellule marcate magneticamente dalla colonna in un tubo come frazione cellulare selezionata positivamente.
NOTA: I volumi per il processo di etichettatura magnetica sono calcolati per un massimo di 1 x 107 celle totali. Se si ottengono più cellule, aumentare di conseguenza tutti i reagenti e i volumi totali. Si raccomanda di lavorare rapidamente e di utilizzare solo soluzioni pre-raffreddate per prevenire il capping degli anticorpi sulla superficie cellulare e la marcatura cellulare non specifica, nonché per garantire un'elevata vitalità delle popolazioni cellulari isolate. È inoltre importante eseguire le fasi di lavaggio non appena il serbatoio della colonna è vuoto aggiungendo il tampone PB in modo che le colonne non si secchino. - Dividere la sospensione cellulare del SNC purificata e non diluita in due frazioni per i seguenti isolamenti di microglia e oligodendrociti. Il rapporto di entrambe le frazioni dipende dal numero di cellule desiderato di ciascun tipo di cellula.
NOTA: Ulteriori dettagli (durata dell'incubazione, fasi dettagliate del protocollo, volumi, reagenti e metodo di conta cellulare) sono indicati nella Tabella 1.
Tabella 1: Flusso di lavoro per la marcatura magnetica simultanea e l'isolamento di oligodendrociti e microglia da topi naïve e EAE. Entrambi i tipi di cellule vengono isolati tramite una selezione positiva. I passaggi elencati nella stessa riga devono essere eseguiti contemporaneamente. Abbreviazioni: CD11b = chinasi ciclina-dipendente 11B; EAE = encefalomielite autoimmune sperimentale; FcR = proteina simile al recettore Fc; O4 =marcatore oligodendrocitario O4. Clicca qui per scaricare questa tabella.
7. Modifica del protocollo: selezione aggiuntiva per l'isolamento della microglia nei topi EAE (Durata: Circa 1,5-2 ore)
NOTA: Quando si lavora con topi EAE, è necessario integrare il protocollo di isolamento cellulare basato su MACS con FACS per rimuovere le popolazioni di cellule CD11b+ diverse dalla microglia (ad es. monociti, macrofagi, cellule natural killer, granulociti o cellule dendritiche) dalla frazione cellulare CD11b+ . In caso contrario, questo passaggio può essere ignorato.
- Preparare la miscela master di colorazione contenente 1x PBS integrato da CD11b FITC (clone M1/70, 1:50) e CD45 APC/Cy7 (clone 30-F11, 1:200). Utilizzare 100 μL della miscela master di colorazione per 5 x 106 cellule. Vortex tutti gli anticorpi prima dell'uso.
- Centrifugare la sospensione di cellule microglia a 300 x g e 4 °C per 10 minuti e aspirare con cura il surnatante.
- Risospendere il pellet cellulare con 100 μL della miscela master di colorazione preparata per 5 x 106 celle. Incubare per 15 minuti al buio a temperatura ambiente (RT).
- Arrestare la reazione aggiungendo 500 μL di PBS e centrifugare nuovamente il campione a 300 x g e 4 °C per 10 min.
- Aspirare con cautela il surnatante e risospendere il pellet cellulare con 1x PBS integrato da 10 μg/mL di DNAsi per raggiungere una concentrazione finale di 1 x 107 cellule per mL. Conservare le cellule a 4 °C fino all'inizio della cernita.
- Applicare la sospensione cellulare su un colino da 100 μm posizionato su una nuova provetta FACS immediatamente prima di iniziare la cernita.
- Impostare la portata a 1000 eventi al secondo e utilizzare l'ugello da 100 μm. Ordinare la popolazione cellulare desideratadi cellule alte CD45intCD11b in una nuova provetta da 15 mL preparata con 1x PBS a RT.
8. Preparazione del flusso negativo di oligodendrociti per l'isolamento di neuroni e astrociti (Durata: 1 ora circa)
NOTA: Il flusso negativo degli oligodendrociti della fase 6 viene raccolto per un ulteriore isolamento di neuroni e astrociti. A tal fine, la sospensione cellulare viene divisa in due parti. A causa del precedente isolamento degli oligodendrociti dalla sospensione cellulare del SNC, la contaminazione da parte delle cellule O4+ è ridotta al minimo rispetto a quanto altrimenti si sarebbe osservato.
- Centrifugare il flusso negativo degli oligodendrociti a 300 x g e 4 °C per 10 minuti e aspirare accuratamente il surnatante.
- Risospendere il pellet cellulare in 80 μL di tampone PB per sospensione di cellule del SNC in pool precedentemente utilizzata per l'isolamento della frazione positiva degli oligodendrociti.
- Conta le celle. Eseguire il conteggio delle cellule che si presume siano O4- utilizzando una camera di conteggio migliorata dopo aver diluito la sospensione cellulare 1:50 in tampone PB seguita da un'ulteriore diluizione 1:10 in blu di tripano allo 0,4%.
- Dividere la sospensione cellulare purificata non diluita in due frazioni per il successivo isolamento simultaneo di neuroni e astrociti. Il rapporto di entrambe le frazioni dipende dalla quantità preferita di ciascun tipo di cellula.
NOTA: Ulteriori dettagli (durata dell'incubazione, fasi dettagliate del protocollo, volumi, reagenti e metodo di conta cellulare) sono indicati nella Tabella 2.
Tabella 2: Flusso di lavoro per la marcatura magnetica simultanea e l'isolamento di neuroni e astrociti da topi naïve e EAE. Entrambi i tipi di cellule sono isolati dal flusso negativo degli oligodendrociti. Gli astrociti vengono separati come una selezione positiva tramite microsfere anti-ACSA-2 mentre i neuroni vengono purificati tramite biotinilazione e deplezione di tutte le cellule non neuronali come selezione negativa. I passaggi elencati nella stessa riga devono essere eseguiti contemporaneamente. Abbreviazioni: Anti-ACSA-2 = antigene di superficie delle cellule astrocitarie-2; EAE = encefalomielite autoimmune sperimentale; FcR = proteina simile al recettore Fc; MACS = selezione cellulare attivata magneticamente. Clicca qui per scaricare questa tabella.
9. Analisi di purezza dei tipi cellulari isolati residenti nel SNC (Durata: 2 ore circa)
NOTA: Si raccomanda di eseguire la citometria a flusso di tutte e quattro le popolazioni cellulari isolate residenti nel SNC per misurarne e confrontarne la purezza e la vitalità. Pertanto, è necessario colorare tutti i tipi di cellule con un anticorpo marcato con fluoroforo. La colorazione delle cellule vive/morte viene implementata utilizzando un colorante di vitalità fissabile (1:10.000).
- Pannello di purezza - protocollo di colorazione extracellulare
- Utilizzare 1 x 105 cellule disciolte in 50 μL di PBS per colorazione.
- Preparare la miscela master di colorazione disciolta in PBS con EDTA FCS/2 mM al 2% costituita dai seguenti anticorpi monoclonali coniugati con fluorocromo che hanno come bersaglio marcatori di superficie specifici per il tipo di cellula: CD11b FITC (clone 1/70, 1:100)25,26,27,28, Biotina-PE (clone Bio3-18E7, 1:200)29,30,31,32, ACSA-2 PE-Vio615 (clone REA-969, 1:200)33,34,35, O4 APC (clone REA-576, 1:400) e CD45 BV510 (clone 30-F11, 1:150)36,37. Aggiungere 1 μg di anti-CD16/32 per 1 x 106 cellule per bloccare il recettore Fc3 8,39. Vortex tutti gli anticorpi prima dell'uso.
- Centrifugare la sospensione cellulare per 5 minuti a 540 x g e 4 °C e aspirare con cura il surnatante.
- Risospendere il pellet cellulare in 100 μL della rispettiva miscela principale e incubare il campione per 15 minuti a RT al buio.
- Lavare le cellule con 500 μL di 1x PBS con 2% FCS/2 mM EDTA e centrifugare il campione per 5 minuti a 540 x g e 4 °C.
- Aspirare il surnatante e risospendere il pellet cellulare con 70 μL di 1x PBS con 2% FCS/2 mM EDTA.
- Vorticare il campione per dissociare completamente il pellet cellulare. Successivamente, il campione è pronto per l'analisi della citometria a flusso.
- Pannello di purezza - protocollo di colorazione intracellulare con NeuN
- Utilizzare 1 x 10-5 cellule di ciascuna popolazione cellulare per la colorazione intracellulare di NeuN, che è un marcatore nucleare neurone-specifico 40,41. Questo è un ulteriore modo per colorare i neuroni vitali.
- Trasferire 1 x 105 cellule di ciascuna popolazione cellulare in una provetta FACS. Aggiungere 1 mL di PBS con 2% FCS/2 mM di EDTA per provetta. Centrifugare le provette a 540 x g e 4 °C per 5 min.
- Nel frattempo, preparare la master mix disciolta in PBS con EDTA FCS/2 mM al 2% costituita dai seguenti anticorpi monoclonali coniugati con fluorocromo che hanno come bersaglio marcatori di superficie specifici per il tipo di cellula: CD11b FITC (clone M1/70, 1:100)25,26,27,28, Biotina-PE (clone Bio3-18E7, 1:200)29,30,31,32, ACSA-2 PE-Vio615 (clone REA-969, 1:200)33,34,35 e CD45 BV510 (clone 30-F11, 1:150)36,37.
- Aspirare il surnatante e risospendere le cellule in 100 μL della miscela master preparata e incubare il campione per 10 minuti a RT al buio.
- Lavare le cellule con 100 μL di PBS con EDTA al 2% FCS/2 mM e centrifugarle nuovamente a 540 x g e 4 °C per 5 min.
- Nel frattempo, preparare 200 μL della soluzione di fissazione/permeabilizzazione: aggiungere 50 μL della scorta concentrata di concentrato di fissazione/permeabilizzazione a 150 μL di diluente di fissazione/permeabilizzazione per ottenere una diluizione finale di 1:4.
- Aspirare il surnatante e risospendere le cellule in 100 μL di soluzione di fissazione/permeabilizzazione 1x. Incubare il campione per 30 minuti a 4 °C.
- Nel frattempo, preparare 1 mL di tampone di permeabilizzazione/lavaggio 1x aggiungendo 100 μL del tampone di permeabilizzazione a 900 μL di ddH2O per ottenere una diluizione finale di 1:10.
- Lavare le cellule 1x con 100 μL di 1x tampone di permeabilizzazione/lavaggio e centrifugare il campione a 540 x g e 4 °C per 5 min.
- Nel frattempo, preparare un'altra miscela master in 1x tampone di permeabilizzazione/lavaggio costituito solo da NeuN (NeuN AF647, clone EPR12763, 1:200) e 1 μg di anti-CD16/32 per 106 cellule per bloccare il recettore Fc.
- Aspirare il surnatante. Risospendere le cellule fisse e permeabilizzate in 50 μL della seconda miscela master e incubare per 30 minuti a 4 °C.
- Lavare il campione con 100 μL di tampone di permeabilizzazione/lavaggio 1x e centrifugare a 540 x g e 4 °C per 5 min.
- Scartare il surnatante e risospendere il pellet cellulare in 70 μL di PBS con 2% FCS/2 mM di EDTA. Successivamente, il campione è pronto per l'analisi citofluorimetrica.
- Dopo aver impostato il pannello sul citometro a flusso, acquisire le cellule per l'analisi della purezza utilizzando un software di analisi della citometria a flusso.
10. Analisi statistica
- Esegui analisi statistiche e progetta grafici con un programma di analisi grafica. I dati sono presentati come media ± SEM.
Risultati Rappresentativi
L'attuale protocollo offre la possibilità di isolare simultaneamente tutte le principali cellule residenti nel SNC, ovvero microglia, oligodendrociti, astrociti e neuroni da una singola replicazione del SNC. Ciò è importante per ridurre il numero di topi necessari per questo tipo di esperimenti e per garantire la comparabilità delle analisi molecolari e biochimiche a livello cellulare. Se i singoli tipi di cellule sono isolati da diverse repliche del SNC, le interazioni cellulari non possono essere mappate in modo veritiero e potenziali deviazioni tecniche durante i processi di isolamento potrebbero influenzare ulteriori analisi a valle. Inoltre, i risultati molecolari e biochimici di ciascun tipo di cellula non sarebbero comparabili tra loro in quanto non derivano dallo stesso contesto EAE. Un protocollo MACS preesistente che utilizzava un sistema/kit commerciale è stato adattato per consentire l'isolamento simultaneo dei tipi di cellule sopra menzionati.
L'isolamento della microglia è stato eseguito utilizzando microsfere anti-CD11b, gli oligodendrociti sono stati isolati tramite microsfere anti-O4 (Tabella 1) e microsfere anti-ACSA-2 sono state utilizzate per isolare gli astrociti (Tabella 2). Al contrario, l'isolamento dei neuroni rappresenta una selezione negativa ed è stato ottenuto mediante biotinilazione e marcatura magnetica di tutte le cellule non neuronali (Tabella 2). Tutte le cellule non neuronali (ad esempio, oligodendrociti, microglia, astrociti, cellule endoteliali e fibroblasti), ad eccezione delle cellule del sangue, possono essere marcate magneticamente utilizzando un anticorpo coniugato con biotina, specificamente diretto contro un antigene di superficie espresso su queste cellule non neuronali (Tabella 2). Mediante l'esaurimento di queste cellule non neuronali marcate magneticamente, è possibile generare popolazioni di cellule neuronali altamente pure e vitali 30,42,43.
Sono stati progettati due nuovi pannelli di citometria a flusso per l'analisi della purezza delle sospensioni monocellulari generate. In questo caso, sono stati utilizzati marcatori di superficie e nucleari specifici per il tipo di cellula combinati con la discriminazione delle cellule vive/morte.
Sono state analizzate le rese cellulari risultanti per topo e tipo di cellula (Figura 3A) che hanno portato a una media di 8. 4 x 105 ± 3 x 104 microglia, 3,23 x 106 ± 1 x 105 oligodendrociti, 8,6 x 105 ± 2 x 104 astrociti e 4,5 x 105 ± 1 x 104 neuroni per topo naïve.
Nell'ambito dell'obiettivo di studiare modelli di malattia di neuroinfiammazione, il protocollo è stato applicato anche a un modello murino di EAE. I topi sono stati soppressi il giorno 16 dopo l'induzione dell'EAE, che rappresentava il massimo della malattia. In questo contesto EAE, sono stati isolati circa 2,9 x 106 ± 6,7 x 105 oligodendrociti, 5,2 x 105 ± 9 x 104 astrociti e 4,4 x 105 ± 4 x 104 neuroni. La produzione di cellule microglia è diminuita a circa 1,7 x 105 ± 4 x 104 microglia per topo EAE a causa dell'ulteriore ordinamento cellulare dopo le fasi MACS (Figura 3A).
Dopo l'isolamento, le caratterizzazioni fenotipiche delle diverse popolazioni cellulari tramite citometria a flusso hanno dimostrato che è possibile ottenere sospensioni vitali a singola cellula con una purezza di circa il 90% per tutti i principali tipi di cellule residenti nel SNC (Figura 3B). Le microglia sono state classificate come CD45intCD11balte come definito in letteratura 44,45,46,47.
Nell'EAE, la microglia doveva essere separata da tutte le cellule CD11b+ per differenziarle da altre cellule immunitarie CD11b+ come monociti, neutrofili, cellule natural killer, granulociti e macrofagi che immigrano nel SNC durante la neuroinfiammazione 27,28,48. Pertanto, le microglia sono state ordinate come cellulealte CD45intCD11 dalla sospensione cellulare CD11b+. L'intera strategia di selezione della microglia è illustrata nella Figura 4. Nei topi naïve, la popolazione di microglia era pari al 91,16% di tutte le singole cellule vive (96% della popolazione totale di CD11b+) (Figura 4A). Nei topi EAE, la popolazione di microglia era pari al 46,85% di tutte le singole cellule vive (55% della popolazione totale di CD11b+) (Figura 4B). Sebbene entrambe le procedure MACS e FACS applichino stress meccanico alle singole cellule, il 75,41% ± l'8,63% delle microglia purificate selezionate erano vitali (Figura 3B).
Gli astrociti e i neuroni che sono stati isolati direttamente dalla sospensione iniziale delle cellule del SNC hanno mostrato una contaminazione rilevante con oligodendrociti, il che ha portato all'ipotesi che l'isolamento simultaneo di neuroni e astrociti dal flusso negativo degli oligodendrociti potesse prevenire questa contaminazione. Le analisi di citometria a flusso hanno confermato che gli astrociti isolati dal flusso negativo degli oligodendrociti avevano una purezza dell'89,23% ± del 2,78% e hanno dimostrato una vitalità dell'80,56% ± dell'1,41%. Analogamente a questi risultati, la purezza dei neuroni isolati dalla frazione di cellule O4- era dell'81,25% ± del 3,31% e la vitalità era dell'83,90% ± del 2,61% (Figura 3B). Questi risultati confermano anche che l'isolamento simultaneo di questi due tipi di cellule solo successivamente all'isolamento degli oligodendrociti non ha alcun impatto sulla quantità di cellule funzionali vitali.
I risultati riguardanti la vitalità e la purezza delle sospensioni unicellulari isolate sono stati molto simili nei topi EAE rispetto a quelli ricevuti nei topi naïve, confermando che questo protocollo è adatto per topi sani e nel contesto dell'EAE (Figura 3B).
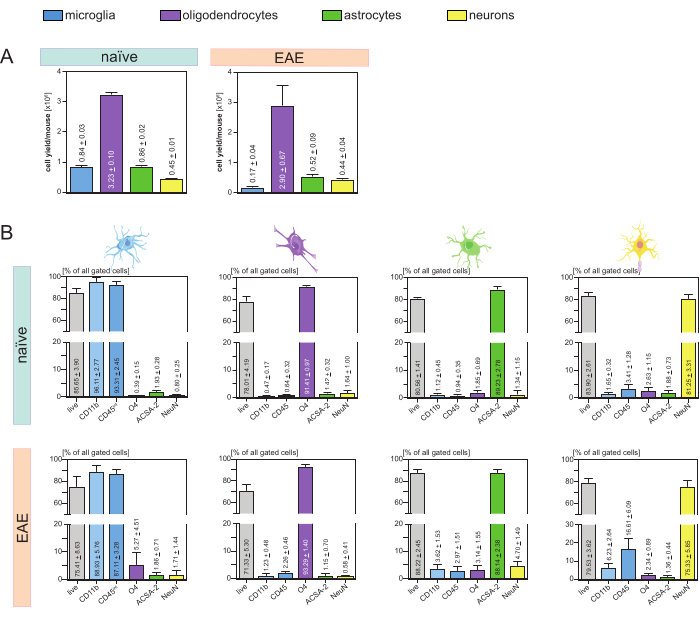
Figura 3: Resa cellulare e validazione basata sulla citometria a flusso di cellule isolate residenti nel SNC. (A) Resa cellulare per topo e tipo di cellula dopo isolamento di cellule residenti nel SNC in topi naïve e EAE. I grafici a barre visualizzano la quantità di rese cellulari per topo e tipo di cellula dopo l'implementazione del protocollo presentato. Cinque repliche biologiche sono state elaborate per i risultati in topi naïve e quattro repliche biologiche sono state analizzate in topi EAE. Vengono rappresentati i rispettivi mezzi ± SEM. (B) Corrispondenti analisi di purezza e vitalità delle frazioni cellulari purificate. I grafici a barre indicano la vitalità e la purezza delle sospensioni a singola cellula risultanti in base alla loro espressione di marcatori specifici del tipo di cellula. NeuN è stato utilizzato come marcatore nucleare specifico per il tipo di cellula per i neuroni. Cinque repliche biologiche sono state acquisite e confrontate per ciascun tipo di cellula sia per topi sani che EAE. Sono indicati i rispettivi mezzi ± SEM. Abbreviazioni: Anti-ACSA-2 = antigene di superficie delle cellule astrocitarie-2; CD11b = chinasi ciclina-dipendente 11B; CD45 = recettore tirosin-proteina fosfatasi C; SNC = sistema nervoso centrale; EAE = encefalomielite autoimmune sperimentale; MACS = selezione cellulare ad attivazione magnetica; NeuN = omologo 3 della proteina legante l'RNA fox-1; O4 = marcatore oligodendrocitario O4; SEM = errore standard della media. Questa cifra è stata modificata da49. Fare clic qui per visualizzare una versione più grande di questa figura.
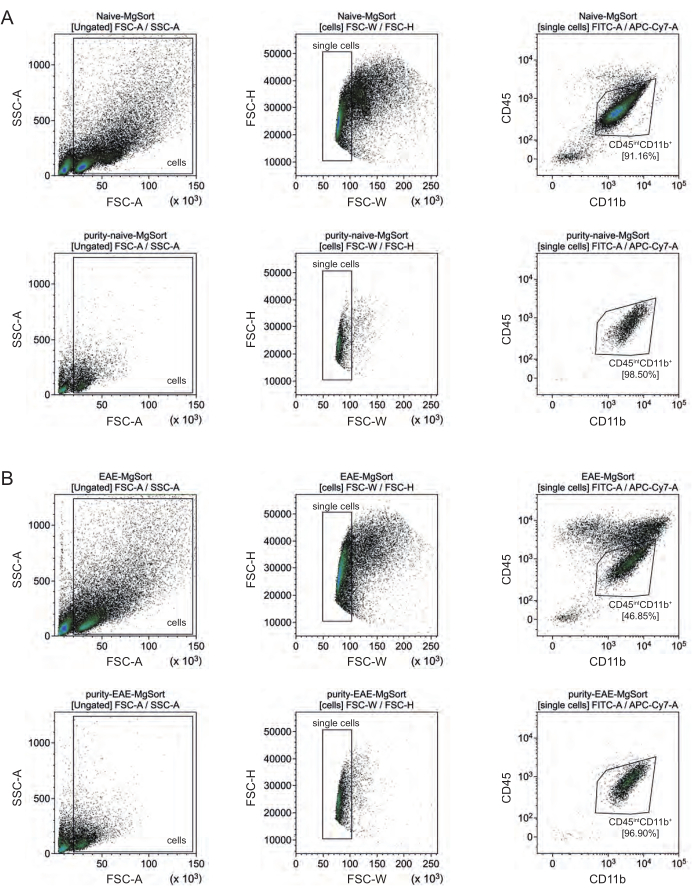
Figura 4: Strategia di gating per lo smistamento cellulare della microglia dopo l'isolamento delle cellule CD11b+. (A) Strategia di gating in topi naïve e (B) EAE. La riga superiore di ciascun pannello mostra i grafici a punti prima dell'ordinamento e la riga inferiore dopo l'ordinamento. Dopo la selezione di cellule vive (SSC-A / FSC-A) e singole (FCS-H / FSC-W), la popolazione di cellule CD45intCD11b+ è stata ordinata come popolazione di microglia. Fare clic qui per visualizzare una versione più grande di questa figura.
Discussione
Finora, i metodi per mappare le cellule residenti nel SNC ex vivo combinando la spettrometria di massa e il sequenziamento dell'RNA offrono un profilo cellulare molto preciso in salute e malattia, ma richiedono conoscenze tecniche e competenze ambiziose in questo campo50,51. Inoltre, non consentono analisi funzionali e sono molto costosi. Oltre a ciò, i sistemi microfluidici brain-on-a-chip forniscono uno screening rapido e conveniente per i meccanismi della malattia e la sperimentazione di nuovi approcci terapeutici con la restrizione della crescita e della migrazione cellulare 52,53,54,55. Gli organoidi del SNC potrebbero anche rappresentare un'alternativa equivalente in futuro per lo studio della modellazione cellulare, delle connessioni intercellulari e delle interazioni durante i decorsi della malattia 56,57,58,59. Tuttavia, la selezione cellulare attivata dalla fluorescenza e dal magnetismo sono attualmente i metodi più efficaci per generare sospensioni monocellulari pure e vitali ex vivo 35,60,61. Anche se altri protocolli di produzione consolidati per l'isolamento dei tipi di cellule residenti nel SNC sono simili per quanto riguarda le singole fasi dell'isolamento magnetico e della precedente dissociazione cellulare, essi sono destinati ad essere eseguiti separatamente per ciascun tipo di cellula. Al contrario, l'attuale protocollo integra diversi metodi di isolamento per ogni tipo di cellula residente nel SNC in un contesto logico in modo che possano essere eseguiti simultaneamente contemporaneamente e da una singola sospensione di cellule del SNC (Tabella 1, Tabella 2). Pertanto, consente analisi multi-omiche da una singola sospensione cellulare del SNC e, infine, l'esplorazione di reti neuronali complesse. Anche se non è necessario raggruppare diversi tessuti di più animali per eseguire questo protocollo, questo raggruppamento garantisce un numero adeguato di cellule isolate per ulteriori analisi a valle. L'utilizzo di topi diversi per l'isolamento dei singoli tipi cellulari escluderebbe la possibilità di analizzare potenziali interazioni cellulari. Oltre a ciò, la combinazione di metodi di isolamento individuali per i diversi tipi di cellule del SNC, che seguono tutti una precedente dissociazione del SNC, consente di risparmiare sui costi dei materiali utilizzando una sospensione di cellule del SNC dissociata per tutte le fasi successive dell'isolamento magnetico. Inoltre, viene ridotta al minimo una potenziale distorsione tecnica causata dall'uso di mouse diversi.
Una limitazione del protocollo potrebbe essere l'uso quasi esclusivo di topi femmina C57BL/6J. Il protocollo di immunizzazione EAE è stato progettato e stabilito per topi femmina, quindi questo protocollo di isolamento cellulare è stato implementato anche nei topi femmina C57BL/6J. Tuttavia, durante lo sviluppo di questo protocollo sono stati utilizzati anche topi maschi naïve, senza riconoscere alcun effetto sul numero di cellule o sulla purezza risultante. Un'altra restrizione riguarda l'isolamento delle cellule magnetiche dei neuroni in quanto non esistono microsfere specifiche per l'isolamento dei neuroni in termini di selezione positiva. Si è ipotizzato che una sospensione unicellulare pura potesse essere ottenuta attraverso la marcatura della biotina e l'esaurimento di tutte le cellule non neuronali (Tabella 2). Questa ipotesi è stata verificata dall'utilizzo di NeuN come marcatore nucleare specifico per i neuroni, integrato nel pannello di purezza della citometria a flusso menzionato. Un'altra limitazione riguarda l'isolamento della microglia nei topi EAE. In questo caso, la resa cellulare risultante è diminuita rispetto agli altri tipi di cellule a causa dell'ulteriore fase di smistamento dopo il protocollo MACS. Inoltre, si potrebbe sostenere che lo smistamento aumenta lo stress meccanico della microglia rispetto alle altre popolazioni cellulari. Le singole strategie di selezione possono portare a diverse quantità di rese cellulari. Se il numero di celle isolate è inferiore a quello previsto o desiderato, si consiglia di regolare l'impostazione del gating e/o di migliorare la discriminazione tra vivi e morti.
Un passaggio critico del protocollo rappresenta la rimozione dei detriti. Il gradiente deve essere stratificato molto lentamente e delicatamente per creare le tre fasi separate desiderate (Figura 2A). Solo se la mielina e gli altri residui di detriti nelle due fasi superiori vengono rimossi completamente (Figura 2E), è possibile generare sospensioni unicellulari pure e ridurre ulteriormente la contaminazione. Se le sospensioni cellulari risultanti mancano di purezza, questa è probabilmente la sezione del protocollo che dovrebbe essere migliorata per prima insieme alla garanzia del corretto utilizzo di tutte le microsfere.
Ricevere alti livelli di purezza e vitalità può essere difficile in questo tipo di esperimento. Di seguito sono riportati alcuni consigli per la risoluzione dei problemi:
-Lavorare in condizioni sterili è obbligatorio per prevenire la contaminazione delle diverse microsfere e consentire un uso ripetuto, soprattutto per la coltivazione successiva.
-Si consiglia vivamente di etichettare ogni tubo per evitare confusioni.
-Evitare l'uso di reagenti/tamponi non raffreddati. Conservare tutte le sospensioni cellulari su ghiaccio durante l'intero esperimento per garantire un'elevata vitalità.
-Mantenere il tempo tra le diverse fasi di lavoro il più breve possibile. Non esiste una parte specifica del protocollo in cui si consiglia di mettere in pausa l'esperimento.
-È molto importante rispettare i periodi di incubazione specificati.
In conclusione, questo attuale protocollo per l'isolamento simultaneo di tutti i principali tipi di cellule residenti nel SNC da una replica del SNC offre la possibilità di analizzare reti neuronali complesse e vie neuroinfiammatorie ex vivo da una sospensione cellulare del SNC. Pertanto, le cellule residenti nel SNC possono essere studiate durante le diverse fasi del decorso della malattia, ad esempio durante la neuroinfiammazione, la neurodegenerazione e/o la remissione nell'EAE. Inoltre, le interazioni cellula-cellula e i percorsi biochimici possono essere studiati a livello individuale e la variabilità all'interno dei gruppi sperimentali può essere ridotta. C'è anche l'opportunità di coltivare frazioni delle cellule isolate del SNC in monocolture per ulteriori saggi funzionali e validazione. Tutto in uno, questo protocollo offre progressi significativi che potenzialmente influenzano gli approcci di ricerca preclinica e clinica.
Divulgazioni
Tutti gli autori dichiarano di non avere conflitti di interesse.
Riconoscimenti
Le figure sono state create utilizzando Adobe Illustrator (versione 2023) e Servier Medical Art (https://smart.servier.com). Antonia Henes è stata sostenuta dalla Jürgen Manchot Stiftung.
Materiali
| Name | Company | Catalog Number | Comments |
| 70 μm cell strainers | Corning, MA, USA | 352350 | CNS tissue dissociation |
| ACSA-2 Antibody, anti-mouse, PE-Vio 615 (clone REA-969) | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-116-244 | Flow cytometry, store at 4 °C |
| Adult Brain Dissociation Kit, mouse, and rat | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-107-677 | Tissue dissociation,contains debris and red blood cell removal solutions; prepare aliquots of enzyme A and P upon arrival and store them at -20 °C; store the remaining kit at 4 °C |
| Anti-ACSA-2 MicroBead Kit, mouse | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-097-678 | MACS of astrocytes, store at 4 °C |
| Anti-mouse CD16/32 antibody | BioLegend, London, UK | 101301 | Flow cytometry, store at 4 °C |
| Anti-O4 MicroBeads, human, mouse, rat | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-094-543 | MACS of oligodendrocytes, store at 4 °C |
| AstroMACS Separation buffer | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-091-221 | MACS of astrocytes, store at 4 °C |
| Biotin Antibody, PE (clone Bio3-18E7) | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-113-853 | Flow cytometry, store at 4 °C |
| BRAND Neubauer counting chamber | Thermo Fisher Scientific,Waltham, MA, USA | 10195580 | Cell counting |
| Brilliant Violet 510 anti-mouse CD45 Antibody (clone 30-F11) | BioLegend, London, UK | 103137 | Flow cytometry, store at 4 °C |
| CD11b MicroBeads, human, mouse | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-049-601 | MACS of microglia, store at 4 °C |
| DNAse I, recombinant, Rnase-free | Merck KGaA, Darmstadt, Germany | 4716728001 | Flow cytometry, store at -20° C |
| D-PBS with Calcium, Magnesium, Glucose, Pyruvat | Thermo Fisher Scientific,Waltham, MA, USA | 14287080 | Buffer, store at 4 °C |
| D-PBS, without calcium, without magnesium | Thermo Fisher Scientific,Waltham, MA, USA | 14190250 | Buffer, store at 4 °C |
| eBioscience Fixable Viability Dye eFluor 780 | Thermo Fisher Scientific,Waltham, MA, USA | 65-0865-14 | Flow cytometry, store at 4 °C |
| eBioscience Foxp3/Transcription factor staining buffer set | Thermo Fisher Scientific,Waltham, MA, USA | 00-5523-00 | Flow cytometry, store at 4°C |
| Falcon (15 mL) | Thermo Fisher Scientific,Waltham, MA, USA | 11507411 | Cell tube |
| Falcon (50 mL) | Thermo Fisher Scientific,Waltham, MA, USA | 10788561 | Cell tube |
| Falcon Round-Bottom Polystyrene Test Tubes with Cell Strainer Snap Cap, 5 mL | Thermo Fisher Scientific,Waltham, MA, USA | 08-771-23 | Flow cytometry |
| FcR Blocking Reagent, mouse | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-092-575 | MACS of oligodendrocytes, store at 4 °C |
| Female C57BL/6J mice | Charles River Laboratories, Sulzfeld, Germany | Active EAE induction | |
| Fetal calf serum (FCS) | Merck KGaA, Darmstadt, Germany | F2442-50ML | Flow cytometry, store at -5 to -20 °C |
| FITC Rat Anti-CD 11b (clone M1/70) | BD Biosciences, San Jose, CA, USA | 553310 | Flow cytometry, store at 4 °C |
| Freund’s Complete adjuvant | Merck KGaA, Darmstadt, Germany | AR001 | Active EAE induction, store at 4 °C |
| GentleMACS C Tubes | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-093-237 | CNS tissue dissociation |
| GentleMACS Octo Dissociator with Heaters | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-096-427 | CNS tissue dissociation |
| Graphpad Prism 8.4.3 | Graphpad by Dotmatics | Graphical Analysis | |
| Isoflurane | AbbVie, North Chicago, IL, USA | Active EAE induction, store at 4 °C | |
| Kaluza Analysis Software V2.1.1 | Beckman Coulter, Indianapolis, IN, USA | Flow cytometry analysis | |
| LS Columns | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-042-401 | MACS |
| MACS BSA Stock Solution | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-091-376 | PB-buffer |
| MACS MultiStand | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-042-303 | MACS |
| MOG35–55 peptide | Charité, Berlin, Germany; alternatives: Genosphere Biotechnologies (Paris, France) or sb-Peptide (Saint Egrève, France) | Active EAE induction, store at -20 °C | |
| Mycobacterium tuberculosis strain H37 Ra | Becton, Dickinson and Company (BD),Franklin Lakes, NJ, USA | Active EAE induction, store at 4 °C | |
| Neuron Isolation Kit, mouse | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-115-390 | MACS of neurons, store at 4 °C |
| O4 Antibody, anti-human/mouse/rat, APC, (clone REA-576) | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-119-897 | Flow cytometry, store at 4 °C |
| Pertussis toxin in glycerol | Hooke Laboratories Inc., Lawrence, MA, USA | BT-0105 | Active EAE induction; store at -20 °C |
| pluriStrainer Mini 100 μm | pluriSelect Life Science UG, Leipzig, Sachsen, Germany | 43-10100-40 | Flow cytometry |
| QuadroMACS Separator | Miltenyi Biotec, Bergisch Gladbach, NRW, Germany | 130-090-976 | MACS |
| Recombinant Alexa Fluor 647 Anti-NeuN antibody (clone EPR12763) | Abcam, Cambridge, UK | EPR12763 | Flow cytometry, store at -20 °C |
| Stainless Steel Brain Matrices, 1 mm | Ted Pella, Redding, CA, USA | 15067 | CNS tissue dissection |
| Trypan blue solution, 0.4% | Thermo Fisher Scientific,Waltham, MA, USA | 15250061 | Cell counting |
| UltraPure 0.5 M EDTA, pH 8.0 | Thermo Fisher Scientific,Waltham, MA, USA | 15575020 | Flow cytometry, store at room temperature |
Riferimenti
- Trapp, B. D., Nave, K. A. Multiple Sclerosis: An Immune or Neurodegenerative Disorder. Annu Rev Neurosci. 31 (1), 247-269 (2008).
- Stys, P. K., Zamponi, G. W., van Minnen, J., Geurts, J. J. Will the real multiple sclerosis please stand up. Nat Rev Neurosci. 13 (7), 507-514 (2012).
- Korn, T. Pathophysiology of multiple sclerosis. J Neurol. 255 (Suppl 6), 2-6 (2008).
- Ward, M., Goldman, M. D. Epidemiology and Pathophysiology of Multiple Sclerosis. CONTINUUM. 28 (4), 988-1005 (2022).
- Bittner, S., Afzali, A. M., Wiendl, H., Meuth, S. G. Myelin Oligodendrocyte Glycoprotein (MOG35-55) Induced Experimental Autoimmune Encephalomyelitis (EAE) in C57BL/6 Mice. J Vis Exp. (86), 51275 (2014).
- Bittner, S., et al. The TASK1 channel inhibitor A293 shows efficacy in a mouse model of multiple sclerosis. Exp Neurol. 238 (2), 149-155 (2012).
- Göbel, K., et al. Plasma kallikrein modulates immune cell trafficking during neuroinflammation via PAR2 and bradykinin release. Proc Natl Acad Sci U S A. 116 (1), 271-276 (2019).
- Ballerini, C. Experimental Autoimmune Encephalomyelitis. Methods Mol Biol. 2285, 375-384 (2021).
- Birmpili, D., Charmarke Askar, I., Bigaut, K., Bagnard, D. The Translatability of Multiple Sclerosis Animal Models for Biomarkers Discovery and Their Clinical Use. Int J Mol Sci. 23 (19), 11532 (2022).
- Tsatas, O., Ghasemlou, N. Isolation and RNA purification of macrophages/microglia from the adult mouse spinal cord. J Immunol Methods. 477, 112678 (2020).
- Calvo, B., Rubio, F., Fernández, M., Tranque, P. Dissociation of neonatal and adult mice brain for simultaneous analysis of microglia, astrocytes and infiltrating lymphocytes by flow cytometry. IBRO Rep. 8, 36-47 (2020).
- Diaz-Amarilla, P., et al. Isolation and characterization of neurotoxic astrocytes derived from adult triple transgenic Alzheimer's disease mice. Neurochem Int. 159, 105403 (2022).
- Galatro, T. F., Vainchtein, I. D., Brouwer, N., Boddeke, E. W. G. M., Eggen, B. J. L. Isolation of Microglia and Immune Infiltrates from Mouse and Primate Central Nervous System. Methods Mol Biol. 1559, 333-342 (2017).
- Altendorfer, B., et al. Transcriptomic Profiling Identifies CD8+ T Cells in the Brain of Aged and Alzheimer's Disease Transgenic Mice as Tissue-Resident Memory T Cells. J Immunol. 209 (7), 1272-1285 (2022).
- Lanfranco, M. F., Sepulveda, J., Kopetsky, G., Rebeck, G. W. Expression and secretion of apoE isoforms in astrocytes and microglia during inflammation. Glia. 69 (6), 1478-1493 (2021).
- Swire, M., Ffrench-Constant, C. Oligodendrocyte-Neuron Myelinating Coculture. Methods Mol Biol. 1936, 111-128 (2019).
- Park, J., Koito, H., Li, J., Han, A. Microfluidic compartmentalized co-culture platform for CNS axon myelination research. Biomed Microdevices. 11 (6), 1145-1153 (2009).
- Facci, L., Barbierato, M., Skaper, S. D. Astrocyte/Microglia Cocultures as a Model to Study Neuroinflammation. Methods Mol Biol. 1727, 127-137 (2018).
- Speicher, A. M., Wiendl, H., Meuth, S. G., Pawlowski, M. Generating microglia from human pluripotent stem cells: novel in vitro models for the study of neurodegeneration. Mol Neurodegener. 14 (1), 46 (2019).
- Homayouni Moghadam, F., et al. Isolation and Culture of Embryonic Mouse Neural Stem Cells. J Vis Exp. (141), 58874 (2018).
- Santos, R., et al. Differentiation of Inflammation-Responsive Astrocytes from Glial Progenitors Generated from Human Induced Pluripotent Stem Cells. Stem Cell Reports. 8 (6), 1757-1769 (2017).
- Tcw, J., et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Reports. 9 (2), 600-614 (2017).
- Miltenyi, S., Müller, W., Weichel, W., Radbruch, A. High gradient magnetic cell separation with MACS. Cytometry. 11 (2), 231-238 (1990).
- Huntemann, N., et al. An optimized and validated protocol for inducing chronic experimental autoimmune encephalomyelitis in C57BL/6J mice. J Neurosci Methods. 367, 109443 (2022).
- Martin, E., El-Behi, M., Fontaine, B., Delarasse, C. Analysis of Microglia and Monocyte-derived Macrophages from the Central Nervous System by Flow Cytometry. J Vis Exp. (124), 55781 (2017).
- Sarkar, S., et al. Rapid and Refined CD11b Magnetic Isolation of Primary Microglia with Enhanced Purity and Versatility. J Vis Exp. (122), 55364 (2017).
- Rodríguez Murúa, S., Farez, M. F., Quintana, F. J. The Immune Response in Multiple Sclerosis. Annu Rev Pathol. 17, 121-139 (2021).
- Engelhardt, B., Ransohoff, R. M. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 33 (12), 579-589 (2012).
- Elia, G. Biotinylation reagents for the study of cell surface proteins. Proteomics. 8 (19), 4012-4024 (2008).
- Berl, S., et al. Enrichment and isolation of neurons from adult mouse brain for ex vivo analysis. J Neurosci Methods. 283, 15-22 (2017).
- Turvy, D. N., Blum, J. S. Biotin Labeling and Quantitation of Cell-Surface Proteins. Curr Protoc Immunol. 18 (7), (2001).
- Mao, S. Y. Biotinylation of Antibodies. Methods Mol Biol. 115, 39-41 (1999).
- Kantzer, C. G., et al. Anti-ACSA-2 defines a novel monoclonal antibody for prospective isolation of living neonatal and adult astrocytes. Glia. 65 (6), 990-1004 (2017).
- Batiuk, M. Y., et al. An immunoaffinity-based method for isolating ultrapure adult astrocytes based on ATP1B2 targeting by the ACSA-2 antibody. J Biol Chem. 292 (21), 8874-8891 (2017).
- Pan, J., Wan, J. Methodological comparison of FACS and MACS isolation of enriched microglia and astrocytes from mouse brain. J Immunol Methods. 486, 112834 (2020).
- Donovan, J. A., Koretzky, G. A. CD45 and the immune response. J Am Soc Nephrol. 4 (4), 976-985 (1993).
- Hathcock, K. S., Hirano, H., Hodes, R. J. CD45 expression by murine B cells and T cells: Alteration of CD45 isoforms in subpopulations of activated B cells. Immunol Res. 12 (1), 21-36 (1993).
- Balogh, P., Tew, J. G., Szakal, A. K. Simultaneous blockade of Fc? receptors and indirect labeling of mouse lymphocytes by the selective detection of allotype-restricted epitopes on the kappa chain of rat monoclonal antibodies. Cytometry. 47 (2), 107-110 (2002).
- Becerril-García, M. A., et al. Langerhans Cells From Mice at Birth Express Endocytic- and Pattern Recognition-Receptors, Migrate to Draining Lymph Nodes Ferrying Antigen and Activate Neonatal T Cells in vivo. Front Immunol. 11, 744 (2020).
- Dent, M. A., Segura-Anaya, E., Alva-Medina, J., Aranda-Anzaldo, A. NeuN/Fox-3 is an intrinsic component of the neuronal nuclear matrix. FEBS Lett. 584 (13), 2767-2771 (2010).
- Duan, W., et al. Novel Insights into NeuN: from Neuronal Marker to Splicing Regulator. Mol Neurobiol. 53 (3), 1637-1647 (2016).
- Monteiro, R., Sivasubramanian, M. K., Balasubramanian, P., Subramanian, M. Obesity-Induced Sympathoexcitation is Associated with Glial Senescence in the Brainstem. FASEB J. 34 (S1), 1-1 (2020).
- Li, S., Chang, L., Teissie, J. . Electroporation protocols: mircroorganism, mammalian system, and nanodevice. , (2020).
- Kettenmann, H., Hanisch, U. K., Noda, M., Verkhratsky, A. Physiology of Microglia. Physiol Rev. 91 (2), 461-553 (2011).
- Haage, V., et al. Comprehensive gene expression meta-analysis identifies signature genes that distinguish microglia from peripheral monocytes/macrophages in health and glioma. Acta Neuropathol Commun. 7 (1), 20 (2019).
- Kosior, N., Petkau, T. L., Connolly, C., Lu, G., Leavitt, B. R. Isolating cells from adult murine brain for validation of cell-type specific cre-mediated deletion. J Neurosci Methods. 328, 108422 (2019).
- Jurga, A. M., Paleczna, M., Kuter, K. Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front Cell Neurosci. 14, 198 (2020).
- Man, S., Ubogu, E. E., Ransohoff, R. M. Inflammatory Cell Migration into the Central Nervous System: A Few New Twists on an Old Tale. Brain Pathol. 17 (2), 243-250 (2007).
- Schroeter, C. B., et al. One Brain-All Cells: A Comprehensive Protocol to Isolate All Principal CNS-Resident Cell Types from Brain and Spinal Cord of Adult Healthy and EAE Mice. Cells. 10 (3), 651 (2021).
- Sankowski, R., et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nate Neurosci. 22 (12), 2098-2110 (2019).
- Brennan, F. H., et al. Microglia coordinate cellular interactions during spinal cord repair in mice. Nat Commun. 13 (1), 4096 (2022).
- Enright, H. A., et al. Functional and transcriptional characterization of complex neuronal co-cultures. Sci Rep. 10 (1), 11007 (2020).
- Mofazzal Jahromi, M. A., et al. Microfluidic Brain-on-a-Chip: Perspectives for Mimicking Neural System Disorders. Mol Neurobiol. 56 (12), 8489-8512 (2019).
- Chin, E., Goh, E. Blood-brain barrier on a chip. Methods Cell Biol. 146, 159-182 (2018).
- Miccoli, B., Braeken, D., Li, Y. E. Brain-on-a-chip Devices for Drug Screening and Disease Modeling Applications. Curr Pharm Des. 24 (45), 5419-5436 (2019).
- Giandomenico, S. L., et al. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci. 22 (4), 669-679 (2019).
- Pellegrini, L., et al. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science. 369 (6500), eaaz5626 (2020).
- Chhibber, T., et al. CNS organoids: an innovative tool for neurological disease modeling and drug neurotoxicity screening. Drug Discov Today. 25 (2), 456-465 (2020).
- Tang, X. Y., et al. Human organoids in basic research and clinical applications. Signal Transduct TargetTher. 7 (1), 168 (2022).
- Sutermaster, B. A., Darling, E. M. Considerations for high-yield, high-throughput cell enrichment: fluorescence versus magnetic sorting. Sci Rep. 9 (1), 227 (2019).
- Doughty, D., et al. Development of a novel purification protocol to isolate and identify brain microglia. Exp Biol Med. 247 (16), 1433-1446 (2022).
Ristampe e Autorizzazioni
Richiedi autorizzazione per utilizzare il testo o le figure di questo articolo JoVE
Richiedi AutorizzazioneEsplora altri articoli
This article has been published
Video Coming Soon