Surgical Model for Single-Staged Tissue-Engineered Urothelial Tubes in Minipigs
In This Article
Summary
Tissue-engineered implants for reconstructive surgery rarely progress beyond preclinical trials due to laborious ex vivo culturing, which includes complex and expensive scaffold components. Here, we present a single-staged procedure designed for urinary diversion with an accessible collagen-based tubular scaffold containing autologous micrografts.
Abstract
Reconstructive surgeries are often challenged by a lack of grafting tissue. In the treatment of urogenital malformations, the conventional solution has been harvesting gastrointestinal tissue for non-orthotopic reconstruction due to its abundance to reestablish normal function in the patient. The clinical outcomes after rearranging native tissues within the body are often associated with significant morbidity; thus, tissue engineering holds specific potential within this field of surgery. Despite substantial advances, tissue-engineered scaffolds have not yet been established as a valid surgical treatment alternative, mainly due to the costly and complex requirements of materials, production, and implantation. In this protocol, we present a simple and accessible collagen-based tubular scaffold embedded with autologous organ-specific tissue particles, designed as a conduit for urinary diversion. The scaffold is constructed during the primary surgical procedure, comprises commonly available surgical materials, and requires conventional surgical skills. Secondly, the protocol describes an animal model designed to evaluate the short-term in vivo outcomes post-implantation, with the possibility of additional variations to the procedure. This publication aims to demonstrate the procedure step-by-step, with special attention to the use of autologous tissue and a tubular form.
Introduction
In urogenital malformations, reconstructive surgery can be required to restore functional anatomy, often on a vital indication1,2. Conventional surgical approaches have utilized native tissues from other organ systems (such as the gastrointestinal tract) to reconstruct the malformed or missing organs; however, often with the risk of severe postoperative complications3,4. In the case of urinary diversion for patients with neurogenic bladder dysfunction in need of long-term catheterization, the appendix or re-tailored small bowel segments are often used to construct a urinary conduit5,6. Tissue engineering offers an alternative grafting tissue that can be tailored to meet organ-specific characteristics, thereby minimizing postoperative morbidity for the patients7,8. Whereas scaffolds of various kinds can be implanted on their own, additional scaffold cellularization, preferably with autologous cells, has been shown to improve the regenerative outcomes after implantation9,10,11,12,13,14. Nevertheless, tissue-engineered scaffolds are often comprised of complex and costly components, and secondly, the requirements for ex vivo cell culturing and scaffold seeding are laborious and resource-intensive. These factors have hindered the clinical translation of tissue-engineered scaffolds despite several decades of research within the area. By reducing the complexity as well as monetary and materialistic requirements, tissue-engineered scaffolds could be implemented in modern surgery on a broad scale, addressing both rare and more common procedures.
Collagen has previously been established as a viable platform for cell expansion and, furthermore, acts as a favorable bio-adhesive when attaching cells or tissue onto a scaffold for surgical implantation15,16,17. Perioperative autologous micrografting circumvents the need for ex vivo cell culturing by harvesting the tissue of interest during the primary procedure and re-implanting it directly. By mincing the resected tissue into smaller particles, the surface area and the growth potential is increased, allowing for a larger expansion ratio onto the scaffold18. The collagen-based scaffold does not adhere specifically to urogenital reconstructions but can theoretically apply to multiple areas of hollow-organ reconstruction.
In this manuscript, we present both a protocol for the construction of a tubular scaffold, combining collagen with embedded autologous urothelial micrografts, and a minipig model evaluating the technical feasibility and safety, as well as the regenerative performance, of the scaffold in vivo. The model was evaluated in 10 full-grown female minipigs using the protocol and method presented here. The main advantage of the scaffold is the simplicity of the construct and the single-staged implantation, sparing the patient of several subsequent surgical procedures. The procedure can be performed in conventional surgical settings by regular surgical personnel and requires standard equipment and materials. The animal model allows for a controlled environment for studying the implantation while the animal readily returns to normal behavior, with the added possibility of implementing variations to the scaffold and the procedure.
Protocol
This experiment was carried out in an AAALAC accredited experimental facility in accordance with the European legislation on laboratory use of animal subjects and after ethical permission granted by the Danish Ministry of Food and Agriculture (Ref. no. 2022-15-0201-01206).
1. Surgical procedure
- Animal preparation
- Fast a female full-grown Göttingen minipig for at least 12 h preoperatively.
- Prepare the surgical table with all sterile utensils as described below.
- For full-grown standard-size minipigs, sedate the animal by intramuscular injection with 1.0-1.4 mL/10 kg with a solution of 125 mg of zolazepam and 125 mg of tiletamine suspended in 1.25 mL of ketamine (100 mg/mL), 6.25 mL of xylazine (20 mg/mL), 1.25 mL of methadone (10 mg/mL) and 2 mL of butorphanol (10 mg/mL) (later on referred as sedation mixture).
- Perform visual-guided endotracheal intubation. Confirm anesthesia by vital signs and eye and interdigital reflex testing. Apply ophthalmic ointment bilaterally.
- Install bilateral ear vein catheters and sustain anesthesia with propofol (10-15 mg/kg/h) and fentanyl (5-15 mg/kg/h).
- Insert an 8 Fr urinary catheter and fill the bladder with 250 mL of physiologically temperate isotonic saline using an appropriately sized luer lock syringe.
- Place the pig in the supine position, then raze and scrub the abdomen. After two further rounds of skin cleaning with 70% ethanol, frame the surgical field with sterile draping.
- Tissue harvesting and surgical scaffold implantation
- Perform a standard lower midline laparotomy with scalpel and cautery, dividing the skin, muscle, and peritoneum, and pull the intraperitoneal urinary bladder to the wound.
- Perform prophylactic hemostasis on the anterior bladder wall and excise a 2 cm2 full-wall segment, leaving a proximal opening of 1 cm2 while closing the remaining bladder wall with a fast-resorbable braided running suture.
- Carefully dissect the mucosal layer of the resected specimen and mince a 2 cm2 mucosal specimen into 1 mm2 micrografts for scaffold embedding (described below in section 2).
- After completing the scaffold, anastomose the tubular construct to the remaining opening on the anterior bladder wall with a slow-resorbable monofilament running suture.
- Use a peritoneal flap from the pubovesical ligament to patch the tubular scaffold and place an intraluminal 14 Fr antegrade colonic enema (ACE) stopper into the tubular scaffold.
- Ligate the distal end of the conduit with a slow-resorbable 4-0 monofilament suture to prevent urine from leaking out, and inject a total of 250 mL of sterile saline with syringes via the bladder catheter to confirm anastomotic patency.
- Bluntly dissect a trans-fascial channel laterally to the midline, 2-3 cm caudally to the caudal mammary gland on the right side, and place the conduit in a subcutaneous pocket. Fixate the distal conduit with two transcutaneous non-resorbable monofilament sutures to mark the location at skin level.
- Close the anterior muscle fascia of the abdominal muscle with a slow resorbable monofilament running suture, adapt the subcutis with a fast-resorbable braided running suture, and close the skin with a non-resorbable monofilament running suture.
- After discontinuing anesthesia, extubate the animal and observe it in the stables until it is fully ambulant and safely able to drink and eat.
2. Scaffold construction
- Preparation of the composite scaffold
- Prior to surgery (maximum 2 h), prepare a liquid solution of rat-tail collagen type I as previously described17. In brief, add 4:1 of 10x minimum essential medium (MEM) to the collagen solution and approximate the pH to 7.4 with 1 M NaOH, and finally add 1x MEM, aiming for a final collagen concentration of 1.64 mg/mL. Store the solution in a sterile vial on ice until further use.
- After surgical tissue resection and mincing, manually place the mucosal particles (i.e., micrografts) onto a 2 cm x 6 cm fitted biodegradable mesh with a 1:6 expansion rate (e.g., a 2 cm2 mucosal tissue is expanded to a 12 cm2 mesh) using forceps.
- Prepare a sterile rectangular steel mold measuring 1 cm x 3 cm x 6 cm (height x width x length) on top of a sterile steel plate and place the mesh into the steel mold with the micrografts facing upwards. Gently pour 20 mL of the collagen solution into the mold, making sure not to flush the micrografts off the mesh. Transfer the entire construct to a 38 °C sterile heating chamber and leave to solidify for five minutes.
- After sufficient solidification, slide the hydrogel onto a nylon mesh resting on a perforated steel plate and gently remove the mold.
- Expel water from the hydrogel by placing a nylon mesh and then a steel plate on top of the gel, and then passively compress with a 120 g weight (in this case equivalent to the steel mold used for embedding) placed on top of the steel plate for 5 min.
- After compression, roll the flattened scaffold around a biodegradable stent, micrografts facing the stent, measuring 5 cm x 0.6 cm (length x inner diameter), and suture the scaffold in place longitudinally with a slow-resorbable monofilament running suture. The completed conduit is now ready for surgical implantation.
3. Postoperative management
- Analgesia and antibiotic prophylaxis
- Administer buprenorphine (0.05-0.1 mg/kg/8 h intravenously) for the first 3 days, meloxicam (0.4 mg/kg/day intramuscular or orally) for the first 4 days, and trimethoprim (2.7 mg/kg/day intramuscular or 4.2 mg/kg/day orally) and sulfadoxin (13.3 mg/kg/day intramuscular or 20.8 mg/kg/day orally) for the first 5 days. Administer the intramuscular injections postoperatively while the animal is still anesthetized.
- Single-house the animals to avoid nibbling of external vein catheters and suture material. Provide visual contact with neighboring minipigs through plexiglass windows and the possibility of snout contact between pens. Provide daily fresh straw and hay, as well as toys and water supply ad libitum and feed twice daily.
- Monitor the animals daily for natural behavior, eating habits, urine- and stool production, and assess body weight weekly.
- At the end of the observational period (6 weeks), sedate the animals with 1-1.4 mL/10 kg intramuscular injection of sedation mixture and terminate the animal with a lethal pentobarbital injection (100 mg/kg intravenously).
4. Postmortem assessments
- Gross anatomy
- After termination, dissect the distal conduit at skin level and remove the ACE stopper. Close the urethra with a plastic clamp and inject 250 mL of a 1:20 contrast solution of iohexol in isotonic saline via the distal conduit opening using a catheter.
- Assess the animal with a 64-slice computed tomography scanner. Visualize images using multiplanar reconstruction and analyze all images using medical image processing software.
- Perform an endoscopic examination of the bladder and conduit lumina with a 16.2 Fr flexible cystoscope via the native urethra.
- Resect the conduit en bloc while carefully evaluating any gross anatomical findings. Additionally, resect full-wall bladder biopsies with a 2 cm margin to the conduit anastomosis and process in a similar fashion for reference values.
- Histological processing
- Fix the excised specimen in 10% formalin for 24 h.
- Divide the conduit orthogonally with a scalpel into equal-sized separate sections of proximal, medial, and distal conduit segments. Dehydrate the specimens with increasing ethanol concentrations and embed them in paraffin before microtome sectioning.
- Stain 5 µm sections with hematoxylin and eosin (H&E) and pancytokeratin CK-AE and scan with a digital histology slide scanner.
Representative Results
In this study, in vivo urothelial tissue expansion is achieved in a collagen-based tubular scaffold. By embedding the scaffold with autologous tissue particles, harvested and processed perioperatively, the procedure allows for single-staged scaffold implantation without the need for concomitant immunosuppressive treatment postoperatively. Surgical handling is enabled by reinforcing the scaffold with a biodegradable mesh and stent (Figure 1). After 6 weeks of observation, the macroscopical tissue evaluation revealed no signs of host rejection or infection, and the tubular scaffold presents patent and unobstructed (Figure 2). From histological evaluations, a stratified luminal epithelium of urothelial origin is seen covering the entirety of the scaffold, and remnants of the reinforcing biomaterials are still visible after 6 weeks (Figure 3).
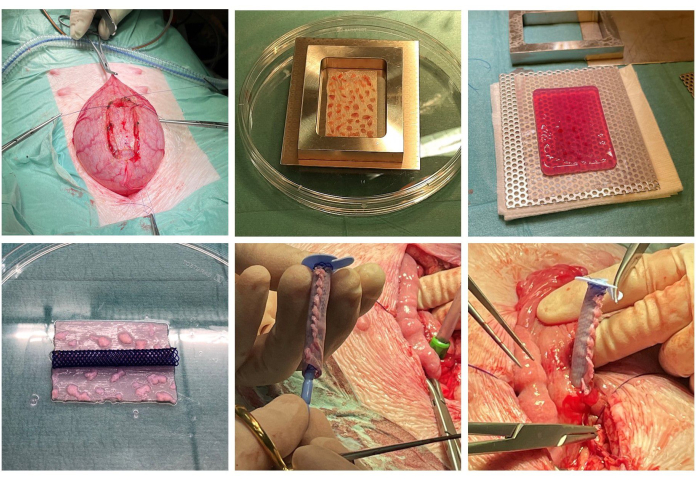
Figure 1: Scaffold construction and implantation. The bladder tissue is dissected perioperatively (top left). The minced mucosal micrografts are expanded onto a surgical mesh (top middle) and embedded in solidified collagen (top right). The collagen has been compressed to expel water, and a stent is prepared (bottom left). The scaffold is tubularized around the stent, and an ACE stopper is placed inside the stent (bottom middle). The bladder is partially closed, and the construct is finally incorporated into the bladder at the original site of tissue excision (bottom right). Please click here to view a larger version of this figure.
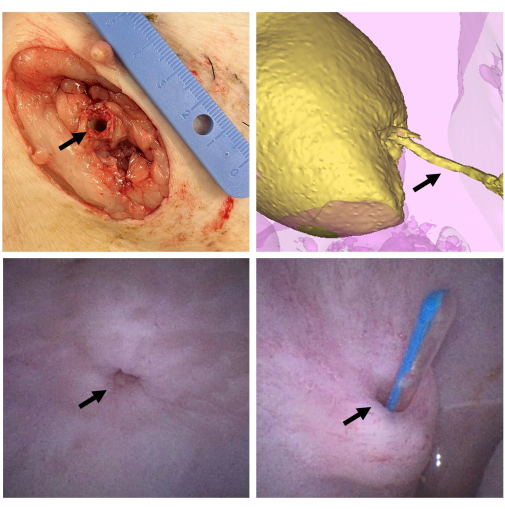
Figure 2: Scaffold macroscopical evaluation. After 6 weeks, the animal is euthanized, and the scaffold (arrow) is dissected at the skin level (top left). The bladder is filled with contrast (yellow) and CT scanning is performed to evaluate the conduit (arrow) for patency and signs of stricture formation (top right). A cystoscopy is performed via the urethra to evaluate the bladder and the anastomosis (arrow) after 6 weeks (bottom left). The conduit is once more tested for patency by inserting a catheter (arrow) via the external opening and into the bladder (bottom right). Please click here to view a larger version of this figure.
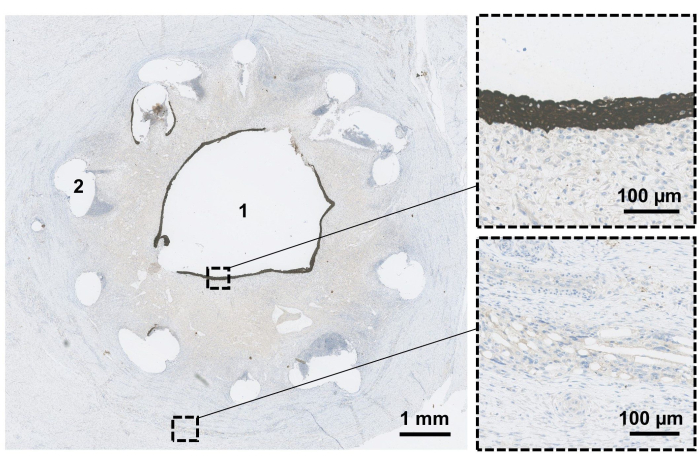
Figure 3: Scaffold microscopical evaluation. The resected conduit is fixated, and orthogonal transverse sections are performed to evaluate the conduit in the proximal-distal direction. After 6 weeks, the conduit lumen (1) is evaluated to confirm epithelialization (magnified top). Remnants of the biodegradable stent (2) and mesh materials (magnified bottom) are still visible at this point. Please click here to view a larger version of this figure.
Discussion
This protocol presents a simple and approachable technique for future reconstructive surgeries. A common drawback in tissue engineering, including autologous cell expansion, is the expensive and substantial prefatory steps required before surgical implantation. Autologous micrografting may simplify many of these steps and potentially allow for single-staged procedures. By auto-transplanting complex histological entities, pro-regenerative paracrine signaling is induced18. In previous studies, we experienced that micrografts alone are vulnerable to the physical environments unless suitably attached to a scaffold15,19. Collagen has been studied as a viable environment for tissue expansion in vitro and was chosen for our purpose due to its favorable biocompatibility and commercial availability. The composite scaffold presented here has previously been optimized during in vitro experiments evaluating variations in micrograft embedding and collagen concentrations20,21,22. Before in vivo testing, the scaffold properties regarding permeability, biomechanics, and degradation have been evaluated in vitro20. Furthermore, the in vivo scaffold-based tissue expansion was previously validated in rodent and rabbit models21,22.
The surgical model was chosen to evaluate a tubular version of the scaffold, mimicking the clinical setting of a urinary diversion for neurogenic bladder dysfunction in pediatric or adolescent patients. The critical steps include the exact dissection of the mucosal micrografts and maintaining a moist environment from the time of resection to the scaffold embedding. Another critical step includes proper hydrogel solidification; careful pipetting of the collagen ensures that air bubbles are not formed within the gel, and correct temperature settings and component solutions ensure that the gel properly solidifies. Failure to obtain a solidified gel will increase the risk of collagen delamination and micrograft detachment. For the surgical part, careful handling during implantation is crucial to avoid damaging the micrografts due to mechanical trauma or dissociation. Before closing the abdomen, fluid patency should be carefully addressed by insufflating the bladder with fluids.
Limitations to the technique include the thickness of the scaffold, which intuitively has upper limits regarding the diffusion of nutrients from the external environment to the micrografts. On the other hand, a reduction in scaffold thickness may lead to inappropriately high permeability and urine leakage. Our current composition is based on previous in vitro assessments, where cell regeneration in varying collagen concentrations was compared20. Micrografting of autologous tissues also relies on healthy graft tissue, making the current procedure unsuited for malignant diseases where the risk of cancerous re-transplantation cannot be properly ruled out23; nevertheless, the current technique was designed for cases with functional voiding disabilities where this is not considered a risk. Although the model mimics several steps from the clinical setting (i.e., the appendicovesicostomy procedure), this experiment does not utilize a fully functional stoma for urinary diversion since the conduit is ligated distally. Also, as clinical complications can occur life-long, a 6 week observational period may provide limited knowledge on specific outcomes on strictures and continency. Therefore, an additional 6-month followup could be added to the study after anastomosing the healed conduit to the skin level.
The perspective of this technique relates to the simple design, enabling universal applications in case the micrograft tissue-origin and supporting biomaterial is replaced with other relevant alternatives. These components can be modified to suit organ-specific purposes related to scaffold strength, elasticity, and biodegradation. Finally, the accessible and low-cost expenses allow for reproducibility and a broadened translation of the technique.
Acknowledgements
The authors would like to acknowledge the staff at the Department of Experimental Medicine (AEM), University of Copenhagen, for assistance with planning and carrying out animal surgeries and husbandry, and ELLA-CS, s.r.o, Hradec Kralove, Czech Republic, for providing customized biodegradable stents used in the study. Financial support was provided by the Swedish Society of Medical Research, Promobilia Foundation, Rydbeck Foundation, Samariten Foundation, The Foundation for Pediatric Health Care, Foundation Frimurare Barnhuset in Stockholm, and the Novo Nordisk Foundation (NNFSA170030576).
Materials
| Name | Company | Catalog Number | Comments |
| 10x MEM | Gibco, Thermo Fisher Scientific, Waltham, US | 2517592 | Collagen preparation |
| 1x MEM | Gibco, Thermo Fisher Scientific, Waltham, US | 2508924 | Collagen preparation |
| Ambu aScope 4 Cysto | Ambu A/S, Ballerup, DK | 1000682507 | Cystoscope |
| Aquaflush ACE stopper | Abena, Taastrup, DK | ACE12/220501 | ACE stopper |
| Borgal vet inj opl 200 + 40 mg/mL | Ceva Animal Health A/S | 510460 | Sulfonamide/Trimethoprim |
| Bupaq multidose vet 0.3 mg/mL | Salfarm Danmark A/S, DK | 502763 | Buprenorphin |
| Butomidor vet inj 10 mg/mL | Salfarm Danmark A/S, DK | 531943 | Buthorphanol |
| Comfortan vet inj 10 mg/mL | Dechra Veterinary Products A/S, DK | 492312 | Metadone |
| Ethilon suture 3-0 | Ethicon, Johnson & Johnson, New Brunswick, US | SGBCXV | Monofilament non-resorbable |
| Fentanyl inj 50 µg/mL(hamel) | Hameln Pharma ApS, DK | 432520 | Fentanyl |
| Ketador vet inj 100 mg/mL | Salfarm Danmark A/S, DK | 115727 | Ketamine |
| Metacam inj 20 mg/mL t.cattle/pig/horse | Boehringer Ingelheim Animal, DE | 6443 | Meloxcicam |
| Metacam oral suspension 15 mg/mL pigs | Boehringer Ingelheim Animal, DE | 482780 | Meloxcicam |
| Omnipaque | GF Healthcare, Oslo, NO | 16173849 | Contrast for CT |
| Pancytokeratin CK-AE | DAKO Agilent, US | GA053 | Clone AE1/AE3 |
| PDS suture 3-0 | Ethicon, Johnson & Johnson, New Brunswick, US | SEMMTQ | Monofilament slow-resorbable |
| Prolene suture 4-0 | Ethicon, Johnson & Johnson, New Brunswick, US | PGH187 | Monofilament non-resorbable |
| Propolipid t.inj/inf 10 mg/mL | Fresenius Kabi, DK | 21636 | Propofol |
| Rat-tail collagen type I | First Link Ltd, Wolverhampton, UK | 60-30-810 | 2.06 mg/mL protein in 0.6% acetic acid |
| Suprim vet 20 + 100 mg (Solution for use in drinking water) | Dechra Veterinary Products A/S, DK | 33661 | Sulfonamide/Trimethoprim |
| SX-ELLA Degradable Biliary DV stent | ELLA-CS, Trebes, CZ | S23000056-01 | ø 6 mm x 60 mm |
| Vicryl mesh | Ethicon, Johnson & Johnson, New Brunswick, US | VM1208 | Mesh |
| Vicryl suture 4-0 | Ethicon, Johnson & Johnson, New Brunswick, US | SMBDGDR0 | Braided fast-resorbable |
| Xysol vet inj 20 mg/mL | ScanVet Animal Health A/S, DK | 54899 | Xylazine |
| Zoletil 50 vet plv/sol t.inj 25 + 25 mg/mL | Virbac Danmark A/S, DK | 568527 | Tiletamine and Zolazepam |
References
- Surer, I., Ferrer, F. A., Baker, L. A., Gearhart, J. P. Continent urinary diversion and the exstrophy-epispadias complex. J Urol. 169 (3), 1102-1105 (2003).
- Cranidis, A., Nestoridis, G. Bladder augmentation. Int Urogynecol J Pelvic Floor Dysfunct. 11 (1), 33-40 (2000).
- Atala, A., Bauer, S. B., Hendren, W. H., Retik, A. B. The effect of gastric augmentation on bladder function. J Urol. 149 (5), 1099-1102 (1993).
- Husmann, D. A. Mortality following augmentation cystoplasty: A transitional urologist's viewpoint. J Pediatr Urol. 13 (4), 358-364 (2017).
- Mitrofanoff, P. Trans-appendicular continent cystostomy in the management of the neurogenic bladder. Chir Pediatr. 21 (4), 297-305 (1980).
- Leslie, B., Lorenzo, A. J., Moore, K., Farhat, W. A., Bägli, D. J., Pippi Salle, J. L. Long-term followup and time to event outcome analysis of continent catheterizable channels. J Urol. 185 (6), 2298-2302 (2011).
- Horst, M., Eberli, D., Gobet, R., Salemi, S. Tissue engineering in pediatric bladder reconstruction-The road to success. Front Pediatr. 7, 91 (2019).
- Ajalloueian, F., Lemon, G., Hilborn, J., Chronakis, I. S., Fossum, M. Bladder biomechanics and the use of scaffolds for regenerative medicine in the urinary bladder. Nat Rev Uro. 15 (3), 155-174 (2018).
- Dorin, R. P., Pohl, H. G., De Filippo, R. E., Yoo, J. J., Atala, A. Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration. World J Uro. 26 (4), 323-326 (2008).
- El Kassaby, A. W., AbouShwareb, T., Atala, A. Randomized comparative study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J Urol. 179 (4), 1432-1436 (2008).
- Casarin, M., et al. Porcine small intestinal submucosa (SIS) as a suitable scaffold for the creation of a tissue-engineered urinary conduit: Decellularization, biomechanical and biocompatibility characterization using new approaches. Int J Mol Sci. 23 (5), 2826 (2022).
- Casarin, M., et al. A novel hybrid membrane for urinary conduit substitutes based on small intestinal submucosa coupled with two synthetic polymers. J Funct Biomater. 13 (4), 222 (2022).
- Drewa, T. The artificial conduit for urinary diversion in rats: a preliminary study. Transplant Proc. 39 (5), 1647-1651 (2007).
- Liao, W., et al. Tissue-engineered tubular graft for urinary diversion after radical cystectomy in rabbits. J Surg Res. 182 (2), 185-191 (2013).
- Reinfeldt Engberg, G., Lundberg, J., Chamorro, C. I., Nordenskjöld, A., Fossum, M. Transplantation of autologous minced bladder mucosa for a one-step reconstruction of a tissue engineered bladder conduit. Biomed Res Int. 2013, 212734 (2013).
- Ajalloueian, F., Nikogeorgos, N., Ajalloueian, A., Fossum, M., Lee, S., Chronakis, I. S. Compressed collagen constructs with optimized mechanical properties and cell interactions for tissue engineering applications. Int J Biol Macromol. 108, 158-166 (2018).
- Chamorro, C. I., Zeiai, S., Engberg, G. R., Fossum, M. Minced tissue in compressed collagen: A cell-containing biotransplant for single-staged reconstructive repair. J Vis Exp. 108, 53061 (2016).
- Juul, N., et al. Insights into cellular behavior and micromolecular communication in urothelial micrografts. Sci Rep. 13 (1), 13589 (2023).
- Reinfeldt Engberg, G., Chamorro, C. I., Nordenskjöld, A., Fossum, M. Expansion of submucosal bladder wall tissue in vitro and in vivo. Biomed Res Int. 2016, 5415012 (2016).
- Juul, N., Ajalloueian, F., Willacy, O., Chamorro, C. I., Fossum, M. Advancing autologous urothelial micrografting and composite tubular grafts for future single-staged urogenital reconstructions. Sci Rep. 13 (1), 15584 (2023).
- Willacy, O., Juul, N., Taouzlak, L., Chamorro, C. I., Ajallouiean, F., Fossum, M. A perioperative layered autologous tissue expansion graft for hollow organ repair. Heliyon. 10 (3), e25275 (2024).
- Chamorro, C. I., et al. Exploring the concept of in vivo guided tissue engineering by a single-stage surgical procedure in a rodent model. Int J Mol Sci. 23 (20), 12703 (2022).
- Casarin, M., Morlacco, A., Dal Moro, F. Bladder substitution: The role of tissue engineering and biomaterials. Process. 9 (9), 1643 (2021).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved