Mechanism of Kemeng Fang's Inhibition of Podocyte Apoptosis in Rats with Membranous Nephropathy through the PI3K/AKT Signaling Pathway
In This Article
Summary
The present protocol describes the establishment of a membranous nephropathy (MN) animal model, and how Kemeng Fang's inhibition reduces MN rat podocyte apoptosis by activating the PI3K/AKT signaling pathway.
Abstract
Membranous nephropathy (MN) is a common pathological type of adult nephrotic syndrome. Up to 20% of patients with MN develop end-stage renal disease (ESRD). Podocytes have an important function in maintaining the glomerular filtration barrier and play a crucial role in the occurrence and development of proteinuria and MN. PI3K/AKT signaling pathway is involved in the entire process of podocyte growth, differentiation, and apoptosis. Kemeng Fang (KMF) is a traditional Chinese medicine formula that has been used to delay kidney injury. However, the therapeutic mechanism of KMF in MN is unclear. Here, the MN rat model was established by axillary, inguinal, and tail vein injections of cationized bovine serum albumin (C-BSA), and then KMF and PI3K inhibitor (LY294002) were administered. The data of liver function, kidney function, blood lipid, renal pathology, podocyte function, expression level of PI3K/AKT signaling pathway, and transcriptomics of rats demonstrated that KMF has a protective effect on the podocytes of MN rats by activating the PI3K/AKT signaling pathway, and it can effectively prevent the progression of MN.
Introduction
Membranous nephropathy (MN) is a common pathological type of adult nephrotic syndrome, with an annual incidence rate of approximately 5-10 per 100,000 individuals. It mostly occurs between the ages of 30 and 50 and is rare in children (about 5%). It is significantly more prevalent in men than women (2:1). Moreover, up to 20% of patients with MN develop the end-stage renal disease (ESRD). Furthermore, there is an increasing trend where patients with MN develop ESRD year by year1,2,3. The pathological feature of MN is that granular immunoglobin G (IgG) and complement system membrane attack complex (MAC) are heavily deposited in the glomerular basement membrane (GBM) adjacent to the podocytes. This deposition leads to the thickening of the GBM and disruption of the glomerular filtration barrier integrity, ultimately leading to proteinuria4.
Supportive therapy, immunosuppressants, and targeted monoclonal antibodies are the main methods for treating MN. Although these interventions can significantly reduce proteinuria and delay the progression of renal deterioration, they also have many shortcomings. First, supportive therapy is only suitable for low-risk patients5. Second, immunosuppressants can cause adverse reactions, such as femoral head necrosis, secondary infection, and inhibition of bone marrow hematopoietic function6. Third, extensive randomized-controlled trial research is needed to provide evidence-based medicine for the use of monoclonal antibodies such as ofamizumab, otuzumab, daretozumab, and isatuximab7,8,9. Therefore, actively seeking effective treatment methods for MN has great significance in delaying the onset of ESRD and improving the quality of life of patients with MN.
Podocytes, also known as glomerular visceral epithelial cells, are attached to the outer side of the GBM, and the GBM capillary endothelium together form the glomerular blood filtration barrier. They have important functions, such as maintaining the glomerular protein filtration barrier, synthesizing normal basement membrane, and providing structural support for the glomerular capillary plexus10,11. Research has shown that apoptosis of podocytes plays a crucial role in the occurrence and development of proteinuria and MN, and the PI3K/AKT signaling pathway is involved in the entire process of podocyte growth, differentiation, and apoptosis12,13,14.
An increasing number of studies have shown that Chinese medicine has significant advantages in the treatment of MN, which can significantly reduce blood creatinine, proteinuria, and delayed kidney injury15,16. KMF is a traditional Chinese medicine compound with ingredients derived from 13 plants: Codonopsis pilosula (Franch.) Nannf. (Dangshen, DS); Astragalus membranaceus (Fisch.) Bunge. (Huangqi, HQ); Coptis chinensis Franch. (Huanglian, HL); Perilla frutescens (L.) Britt. (Suye, SY); Rehmannia glutinosa (Gaertn.) DC. (Shudihuang, SDH); Ligusticum chuanxiong Hort. (Chuanxiong, CX); Euryale ferox Salisb. (Qianshi, QS); Sabia japonica Maxim. (Qinfengteng, QFT); Rhus chinensis Mill. (Wubeizi, WBZ); Lobelia chinensis Lour. (Banbianlian, BBL); Oldenlandia diffusa (Willd.) Roxb. (Baihuasheshecao, BHSSC; Table 1). KFM has many functions, such as tonifying the kidney (enhancement of renal function), enhancing qi (strengthening immunity), promoting diuresis, and dredging collaterals (promoting blood circulation). However, the therapeutic mechanism of KMF in MN is unclear17,18.
At present, there are many ways to construct MN models, Including Heymann nephritis model, C-BSA nephritis model, α3NC1 mouse model, in which Heymann nephritis model, the main pathogenic antigen megalin protein is not found in human MN, so it is different from the pathogenesis of human MN, α3NC1 mouse model, only DBA/1 genetic background of the mouse model success rate is higher, the rest of the mice were less successful in modeling, or even unable to be modeled19,20,21. The C-BSA nephritis model is cost-effective and simple to operate, and its pathogenesis is highly similar to that of the human MN animal model19. The basic principle is that because the GBM is negatively charged, and C-BSA is positively charged, it can easily cross the GBM to become a planted antigen, which induces circulating antibodies to accumulate there to form an in situ immune complex, thereby constructing an MN model22,23. The aim of this study was to observe the therapeutic effect of KMF on MN and its molecular mechanism by a combination of transcriptomics and molecular biology and to provide a reliable scientific basis for the treatment of MN with KMF.
Protocol
This study was reviewed and approved by the Experimental Animal Management and Use Committee of the Hubei Provincial Center for Disease Control and Prevention (ID number: 202220144). The rats underwent a 12 h light/dark cycle under non-pathogenic conditions of 23 ± 1 °C and 50%-60% atmospheric humidity. We procured 100 male 8-week-old Sprague-Dawley rats from the Hubei Provincial Center for Disease Control and Prevention (license number: SYXK [E] 2022-0065), and they were subjected to adaptive feeding in a specific pathogen-free grade environment for 1 week with a normal maintenance feed and drinking sterile water.
1. Drug preparation
- Preparation of KMF
- The Chinese medicines used were provided by the hospital affiliated to Changchun University of Traditional Chinese Medicine, the specific composition and dosage are shown in Table 1. To prepare, use a total of 147 g of raw medicines-Codonopsis pilosula (20 g), Astragalus membranaceus (30 g), Coptis chinensis (3 g), Perilla frutescens (6 g), Rehmannia glutinosa (15 g), Ligusticum chuanxiong (15 g), Euryale ferox (15 g), Sabia japonica (10 g), Rhus chinensis (3 g), Lobelia chinensis (15 g), Oldenlandia diffusa (15 g). Mix and soak in 1470 mL of distilled water for 30 min.
- Place the herbal mixture in a ceramic pot and heat at 100 °C for 90 min for decoction. Filter through two layers of medical gauze and store at room temperature.
- Add distilled water (1470 mL) again to the leftover herbal mixture and repeat the above decoction operation once more, as described in step 1.1.2. Store the filtrate at room temperature.
- Mix the above two filtrates and oven-dry at 80 °C for 10 h until the aqueous solution has completely evaporated and only the solute is retained in the form of powder.
- Weigh the powder and dissolve it in saline to make a solution containing 1.323 g, 2.646 g, and 5.292 g of the drug per 4 mL (the concentrations were 0.331 g/mL, 0.662 g/mL, and 1.323 g/mL, respectively), which is the daily dosage of the drug administered to rats.
- Preparation of Benadryl hydrochloride
- Add Benadryl hydrochloride tablet (90 g) into 100 mL of saline and shake well to completely dissolve.
- Preparation of PI3K inhibitor LY294002
- Add 82.3 mg of LY294002 powder into 1.1 mL of DMSO and shake well to completely dissolve it. Next, add 198.9 mL of saline to dilute it to obtain a concentration of 0.41 mg/mL.
- Preparation of C-BSA emulsifier
- Add 67 mL of anhydrous ethylenediamine to 500 mL of double-distilled water and mix. Slowly add 350 mL of 6 M hydrochloric acid and adjust the pH to 4.75. Maintain the temperature of the final solution at 25 °C.
- Dissolve 5 g of natural bovine serum albumin in 25 mL of double-distilled water and maintain the solution at a constant temperature of 25 °C with constant stirring. Add 1.8 g of carbodiimide hydrochloride and 30 mL of 4 M acetic acid buffer with pH 4.75 after 2 h to obtain the C-BSA solution.
- Dialyze the obtained solution in double-distilled water at 4 °C for 72 h (with the water changed every 3-5 h) using a selenite paper and freeze-dry to obtain C-BSA lyophilized powder, Store at -80 °C24.
- Add 100 mg of C-BSA dried powder in 50 mL of saline to form a C-BSA solution. Mix this with an equal volume of Incomplete Freund's adjuvant for complete emulsification; its concentration is 1 mg/mL.
- Preparation of C-BSA solution
- Add 640 mg of C-BSA dry powder in 100 mL of PBS and shake well to fully dissolve it; its concentration is 6.4 mg/mL.
2. Establishment of MN animal model
NOTE: The experiment was divided into eight groups: normal control group (CON), model group (MOD), benazepril hydrochloride group (BEN), KMF low-dose group (KM-L), KMF medium-dose group (KM-M), KMF high-dose group (KM-H), PI3K inhibitor group (PI3K), and PI3K inhibitor + KMF medium-dose group (PI3K + KM-M). Except for the normal control group, all groups were administered C-BSA to produce the MN model.
- At the ninth week perform pre-immunization: Multiple subcutaneous injections of C-BSA emulsifier in the axilla and groin.
NOTE: The scruff of the neck (interscapular) is as an alternative site for subcutaneous administration of C-BSA emulsifier.- Grasp the back skin of the rats with the left hand and turn their abdomen upward with the abdominal skin tightened. Inject the C-BSA emulsifier subcutaneously into the axillae and groin of the rats using a 2.5 mL syringe at the dose of 1 mL/400 g once every other day for 1 week.
- Formal immunization: Tail vein injection of C-BSA solution
- At the tenth week, remove the rats from their cages and place them on the wire bar lid with their tails facing the experimenter.
- Wipe the rat's tail with an alcohol cotton ball and pinch both sides of the rat's tail with the thumb and forefinger of the left hand to fill the vein and keep the vein facing up.
- Hold a 1 mL syringe in the right hand so that the needle is at 30° to the tail vein. Keep the tip of the needle beveled upward and the needle parallel to the vessel immediately after gently pricking into the skin. If there is any return of blood from the needle, inject it back along with a C-BSA dose of 2.5 mL/kg 3x a week for 4 weeks.
- After completion of the injection, use a dry cotton ball to press the injection point for approximately 1 min to stop the bleeding. Remove the mice from the immobilizer and return to the cage.
3. Analysis of KMF
- Weigh 1 g sample into a 2 mL centrifuge tube, add 600 µL of MeOH (stored at -20 °C containing 2-Amino-3-(2-chloro-phenyl)-propionic acid (4 ppm)), and vortex the mixture for 30 s.
- Add approximately 100 mg of glass bead and place the mixture in a tissue grinder for 90 s at 60 Hz.
- Perform ultrasound at 40 kHz for 15 min at room temperature.
- Centrifuge for 10 min at 15,984 x g, 4 °C, and filter the supernatant through a 0.22 µm membrane. Transfer a detection bottle for LC-MS detection.
4. Drug treatments
NOTE: Adult humans need 147 g KMF per day. According to the conversion formula of experimental rat and human drug dose, the equivalent experimental dose for rat (g/kg) = human dose (g)/body weight (70 kg) x 6.3, the daily dose of the rat was approximately 13.23 g/kg.
- At the 14th week, using the left hand, grasp the skin on the back of the rats, turn their abdomen upward, and tighten the abdominal skin. Hold the needle of a 10 mL syringe in the right hand and insert it into the mouth from one side, sliding along the palate and the posterior wall of the pharynx and further along to the stomach using the swallowing action.
- Administer the drug slowly using the index finger of the right hand and pull the gastric needle out after the administration is complete. The dosage of the administered drug is 10 mL/kg for a total duration of 4 weeks.
- For the CON and MOD groups, administer 10 mL/kg/day saline by gavage.
- For the BEN group, administer 9 mg/kg/day Benadryl hydrochloride aqueous solution by gavage.
- For the KM-L group, administer 3.3075 g/kg/day aqueous extract of KMF by gavage. For the KM-M group, administer 6.615 g/kg/day aqueous extract of KMF by gavage. For the KM-H group, administer 13.23 g/kg/day aqueous extract of KMF by gavage.
- For the PI3K group, administer 2.1 mg/kg/day LY294002 by gavage.
- For the PI3K+KM-M group, administer 2.1 mg/kg/day LY294002 (5 mL/kg) + 6.615 g/kg/day KMF aqueous extract (5 mL/kg) by gavage.
5. Evaluation of KMF efficacy
- Detection of blood and urine biochemical indices
- From the 14th week onwards, collect urine every 2 weeks after the start of the experiment. Place a single rat in a metabolic cage for 24 h. Restrict diet and provide free access to drinking water. Collect urine using a 50 mL centrifuge tube placed below the metabolic cage.
- Centrifuge the urine at 15,984 x g for 10 min and use the supernatant to estimate the level of urinary protein according to the instructions of the Urinary Protein Test Kit.
- At the 18th week, euthanize rats by inhalation of 5% isoflurane. Use vet ointment on eyes to prevent dryness. Confirm death using the cervical dislocation method. Grasp the skin on the back of the rats with the left hand and trim the whiskers of the rats with scissors.
- Disinfect the skin around the eyeballs with ethanol and quickly remove the eyeballs using hemostatic forceps. Collect the blood dropped into the centrifuge tube and centrifuge for 10 min at 15,984 x g after standing at room temperature for 30 min. Collect the supernatant.
- Detect Alanine aminotransferase (ALT), Aspartate transaminase (AST), Albumin (ALB), Triglyceride (TG), Total cholesterol (TC), Blood urea nitrogen (BUN), Serum creatinine (Scr), TotalProtein (TP) and other blood indices using an automatic biochemical analyzer.
- Separation of renal tissues
- After the blood is taken from the rats, fix them on the surgical plate, and slowly cut the fur and muscular layer at the midline of the abdomen, pushing the intestinal tubes open to expose the abdominal aorta and kidneys25.
- Block the abdominal aorta above the right kidney using surgical artery clamps. Attach a scalp needle to a 5 mL syringe to puncture below the block and perfuse PBS buffer to irrigate the kidneys until both kidneys become white or pale in color.
- Remove the kidneys and bluntly separate the periosteum. Divide the right kidney into freeze-storage tubes and store in the refrigerator at -80 °C. Fix the left kidney in 4% paraformaldehyde solution.
- After 24 h of fixation, remove the kidney. Perform dehydration, transparency, and paraffin embedding. After the wax block solidifies, slice with a tissue slicer at a thickness of 3 µm, fish out with an anti-dehiscence slide, bake the slices at 98 °C for 20 min, and store for spare26.
- Renal histopathological analysis
- Subject the sections to gradient xylene dewaxing and anhydrous ethanol dehydration and rinse using distilled water. Subject to xylene for 15 min, followed by fresh xylene solution for 15 min. Switch to anhydrous ethanol for 5 min, change to fresh anhydrous ethanol for 5 min, followed by 90% ethanol for 5 min, then 80% ethanol for 5 min, then 70% ethanol for 5 min, and finally flush with distilled water.
- Follow the instructions for hematoxylin-eosin staining (H&E), periodic acid-Schiff (PAS), and Masson staining kits for sample staining.
- Perform gradient anhydrous ethanol dehydration and xylene clear again.
- Place the slices in a constant temperature incubator, set the temperature to 90 °C, and bake for 20 min. After baking, remove the slices and add neutral gum dropwise. Cover them with a cover glass.
- Observe under a light microscope at a magnification of 200x and collect images.
- Immunofluorescence (IF) analysis of renal tissues
- Dewax sections and dehydrate as described in step 5.3.1. Place samples in 0.01 M EDTA buffer solution for high-pressure, high-heat (125 °C at 103 KPa) fixation for 15 min. Let the sample cool naturally, and wash 3x for 3 min each in PBS.
- Place the sections in a 3% hydrogen peroxide solution and incubate for 10 min. Wash the samples 3x for 5 min each in PBS. Then, place the sections in 10% goat serum, incubate for 30 min, and wash 3x for 3 min each in PBS.
- Add primary antibody IgG (1:100) and C3 (1:100) dropwise and incubate in a wet box at 4 °C overnight. The primary antibody dilution ratio is shown in Table 2. Remove the sections from the refrigerator and wash with TBST 3x for 3 min each.
- Add fluorescent secondary antibody (1:100) dropwise, incubate at 37 °C for 1 h, and wash with TBST 3x for 3 min each.
NOTE: This step and all subsequent steps should be carried out in the dark27,28. - Incubate with 4',6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature and avoid light, wash with tris-buffered saline with tween 20 (TBST) 3x for 5 min each to remove excess DAPI.
- Add autofluorescence quencher in a circle, incubate for 5 min, and rinse with running water for 10 min. Shake dry the sections and seal with anti-fluorescence quenching sealer. Observe under a fluorescence microscope at 200x and collect images.
- Immunohistochemical (IHC) analysis of kidney tissue
- Dewax sections and dehydrate as described in step 5.3.1. Repair under high pressure (125 °C · 103KPa) in 0.01M EDTA buffer solution for 15 minutes. After natural cooling, wash with PBS 3x for 3 min each.
- Block endogenous peroxidase and serum sealing operation method as described in step 5.4.2.
- Add primary antibody WT-1 (1:200) and Nephrin (1:100) dropwise and incubate in a wet box in the refrigerator at 4 °C overnight29.
- Remove the sections from the 4 °C refrigerator, wash with PBS 3x for 5 min each. Add secondary antibody (1:200) dropwise, incubate at 37 °C for 30 min, and wash with PBS 3x for 3 min each.
- Add drops of freshly prepared diaminobenzidine (DAB) chromogenic solution and observe under the light microscope at 200x. The positive signal is brownish yellow or brownish brown. Wash the staining solution immediately with tap water when a color change in the section is observed.
- Use hematoxylin to restain for 3 min, then add 1% hydrochloric acid alcohol for differentiation and rinse with tap water for 10 min.
- Dehydrate the sections, transparent, and seal, as described in step 5.2.4. Observe under the light microscope at 200x and collect images.
- Analysis of terminal deoxynucleotidyltransferase-mediated dUTP nick-end Labeling (TUNEL) staining of renal tissue
- Use step 5.3.1 to deparaffinize and dehydrate the sections. Draw circles around the tissues using an immunohistochemistry pen and add 100 µL of Proteinase K working solution dropwise to each sample. Incubate at 37 °C for 20 min, wash with PBS 3x for 5 min each, and store the treated samples in a wet box.
- Place the sections in 3% hydrogen peroxide solution, incubate for 10 min, and wash with PBS 3x for 5 min each.
- Add equilibrium buffer (100 µL) dropwise to the circle to cover the tissue and incubate for 20 min at room temperature.
- Add 50 µL of labeling working solution (equilibrium solution: fluorescent labeling solution: TDT enzyme = 35 µL:10 µL:5 µL) dropwise to each sample, incubate in a wet box at 37 °C for 60 min, protected from light, and wash with PBS 3x for 5 min each.
- Add DAPI and incubate with DAPI for 5 min at room temperature under light, wash with PBS 3x for 5 min each.
- After the sections were shaken dry and sealed with anti-fluorescence quenching sealer, observe under a fluorescence microscope at 200x and collect images.
- Sample preparation for electron microscopic analysis of renal tissue
- Fix the kidney tissues with 2.5% glutaraldehyde at 4 °C for 2-4 h and wash 3x for 15 min each in PBS.
- Fix in osmium acid (1%) at room temperature away from light for 2 h and wash with PBS 3x for 15 min each.
- Perform gradient alcohol and acetone dehydration, using acetone and embedding agent Immerse in 1:1, 1:2, and 1:31 gradients for 2 h, 4 h, and 8 h, respectively. After this, use pure embedding agent and embed the samples at 37 °C overnight.
- After 8 h, place the embedded plate in a 60 °C oven for polymerization for 48 h. Slice the resin block processed above in an ultra-thin slicer, with a slice thickness set to 60-80 nm; use copper mesh to dislodge slices.
- Use a 2% hydrogen peroxide acetate and alcohol solution for staining for 8 min, followed by a 70% ethanol and ultrapure water wash 3x. Complete by washing with 2.6% ethanol and ultrapure water.
- Observe and acquire images under a transmission electron microscope.
- Quantitative real-time polymerase chain reaction (qRT-PCR) assay analysis
- Add 20 mg of frozen kidney tissue to the microcentrifuge tube and grind thoroughly with a high-speed tissue grinder until there is no visible tissue mass.
- Collect the supernatant after centrifugation at 15,984 x g for 10 min. Add 250 µL of chloroform and invert the tube for 15 s; the solution is mixed thoroughly. Leave to stand at room temperature for 3 min, then centrifuge at 15,984 x g for 10 min at 4 °C.
- Collect the upper aqueous phase and transfer to a new RNase Free centrifuge tube. Add an equal volume of 70% ethanol (RNase-free water preparation), invert, and mix.
- Add the solution to the adsorbent column loaded into the collection tube for RNA washing. After Buffer RW washing, leave the column at room temperature for 5 min to dry.
- Place the column in a new RNase-free centrifuge tube, add 30-50 µL of RNase-free water to the middle of the column, allow to stand at room temperature for 1 min, and then centrifuge at 15,984 x g for 1 min. Collect the RNA solution and store it at -80 °C.
- Remove the RNA solution from the freezer and pre-denature at 90 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 35 s, and 72 °C for 25 s, and finally lysed in the order of 95 °C for 15 s, 60 °C for 60 s, 95 °C for 15 s (Supplementary Table 1).
- Using β-actin as an internal reference, calculate the relative expression of WT-1 and Nephrin using the 2¬-ΔΔCt method based on the Ct value of each sample (ΔΔCt = Ct value of target gene - Ct value of internal reference gene)30. Detailed information on primers is shown in Table 3.
- Western blot (WB) analysis
- Prepare the lysate according to the ratio of protease inhibitor:phosphatase inhibitor:RIPA= 1:1:100, and add 500 µL of lysate into a microcentrifuge tube.
- Add 100 mg of frozen kidney tissue to the microcentrifuge tube and grind thoroughly with a high-speed tissue grinder until there is no visible tissue mass.
- Remove the microcentrifuge tube, lyse on ice for 30 min, and then centrifuge at 15,984 x g for 10 min. Aspirate the supernatant and store it at -80 °C.
- Detect the protein concentration according to the instructions of the bicinchoninic acid (BCA) Protein Concentration Assay Kit and add PBS solution dropwise to ensure consistent protein concentration in each group.
- Add 5x protein up-sampling buffer to the microcentrifuge tube, incubate at 100 °C for 15 min to fully denature it, and remove after cooling.
- Separate the proteins by 12.5% SDS-PAGE gel electrophoresis, first using 80 V, causing all the samples to be pressed into a flat blue line, and then adjusting the voltage to 130 V, until the bromophenol blue runs out from the bottom of the gel plate. Stop the electrophoresis.
- Remove the gel. Wet the filter paper with membrane transfer buffer. Activate the polyvinylidene fluoride (PVDF) membrane in methanol for 30 seconds. Then, arrange everything in the following order: sponge mesh/filter paper/gel/PVDF membrane/filter paper/filter paper/sponge mesh in the clip used for transferring the membrane.
- Place the gel in the negative pole, the PVDF membrane in the positive pole, and transfer the membrane with a current of 200 MA for 90 min.
- Remove the PVDF membrane and wash with TBST once for 5 min. Add the closure solution at room temperature while shaking for 90 min. Wash with TBST 3x for 5 min each.
- Add primary antibodies PI3K (1:1000), PIK3CA (1:1000), AKT (1:1000), P-AKT (1:1000), BAD (1:1000), P-BAD (1:1000), BCL-2 (1:1000), bax (1:4000), and c-caspase3 (1:1000) and incubate in a wet box overnight at 4 °C in the refrigerator31,32.
- Remove the PVDF membrane from the 4 °C refrigerator, wash with TBST 3x for 5 min each. Add secondary antibody (1:10,000) dropwise, incubate at 37 °C for 90 min, and wash with TBST 3x for 3 min each.
- Prepare the developing solution according to the ratio (A: B=1:1) and develop them on the machine as per the instructions.
Representative Results
Analysis results of the components of KMF
In the positive and negative ion modes analyzed by LC-MS/MS, 147 and 120 compounds were identified, respectively (Figure 1A-B). Some compounds and their MF-calculated molecular weight, m/z value, retention time, and parent ions are shown in Supplementary Table 2.
KMF improved lipid metabolism disorders and liver and kidney injury in MN rats
Using SD rats and C-BSA, we established an MN model. After 1 week of tail vein injections of C-BSA, the MN rats exhibited varying degrees of mental fatigue, decreased appetite, slow growth, dull hair color, fluffy fur, delayed response, and weight loss, with some rats developing scrotal edema. After 4 weeks of administration, different doses of KMF significantly reduced the expression levels of 24 h urinary total protein (24 h-UTP), Scr, and BUN, enhanced renal function (Figure 2A-C); reduced ALT and AST expression levels and increased TP and ALB expression levels, enhance liver function (Figure 2D-G); reduced the expression levels of TC and TG, and improved the function of lipid regulation (Figure 2H-I). These results suggest that KMF has a protective effect on renal function; however, its specific mechanism of action is not yet clear.
KMF improves histopathological damage of renal tissue in MN rats
To test whether KMF could improve renal injury in MN rats, using H&E, PAS, Masson, and IF detected histopathological damage of renal tissue. H&E and PAS staining showed glomerular hypertrophy, mild proliferation of mesangial cells, renal tubular dilation, and vacuolar degeneration of renal tubular epithelial cells in the MOD group (Figure 3A). Masson staining showed a significant increase in the area of renal fibrosis in the MOD group (Figure 3B-C). Immunofluorescence showed that the relative fluorescence intensity of IgG and C3 in the MOD group was significantly higher than that in the CON group (Figure 3D-F). After administering KMF, BEN, or PI3K inhibitors+KM-M, the degree of glomerular hypertrophy, as well as the degree of proliferation of tethered cells, was reduced, the area of renal fibrosis was significantly reduced, and the relative fluorescence intensity of IgG and C3 was significantly lower. These results indicate that KMF can alleviate renal pathological damage in MN rats.
KMF alleviates podocyte damage by activating the PI3K/AKT signaling pathway
The damage of key podocytes in MN was also observed using methods described here, namely IHC, PCR, TUNEL, and TEM. IHC and PCR results showed that compared with the CON group, the MOD group had a significant decrease in the expression levels of podocyte-specific and functional marker proteins, WT-1 and Nephrin, indicating podocyte damage, while KMF treatment increases the expression levels of WT-1, Nephrin and alleviates podocyte damage (Figure 4A-E). The TUNEL staining results showed severe apoptosis of podocytes in the MOD group, while KMF treatment significantly decreased fluorescence intensity and reduced the incidence of apoptosis (Figure 4F-G). The TEM results showed that the basement membrane of the glomerulus in the MOD group was significantly unevenly thickened, and the mitochondria of podocytes showed severe swelling, sparse matrix, and empty bombardment of the matrix, with reduced or absent cristae. After administering KMF, the thickening of the glomerular basement membrane was significantly reduced, and the morphology of podocyte mitochondria was significantly restored (Figure 4H). These results indicate that KMF can alleviate podocyte damage in MN rats.
Further detection of the expression of PI3K/AKT signaling pathway-related proteins26,27 by WB revealed that compared with the CON group, the MOD group showed a significant increase in the expression levels of PI3K, PIK3CA, AKT, P-AKT, BAD, BAX, and C-caspase3, while the expression levels of P-BAD and BCL-2 decreased significantly. While KMF treatment reduces the expression levels of PI3K, PIK3CA, AKT, P-AKT, BAD, BAX, and C-caspase3, it increases the expression levels of P-BAD and BCL-2 (Figure 4I-J). These results further indicate that KMF can improve podocyte damage in MN rats by activating the PI3K/AKT signaling pathway.
Exploring the possible mechanisms of KMF therapy for MN based on transcriptomics
To further reveal the targets and potential mechanisms of KMF in treating MN, a transcriptomic analysis was conducted based on Tandem Mass Tag (TMT). The results showed that there were 898 differentially expressed genes (DEGs) between the CON and MOD groups, including 372 upregulated and 526 downregulated genes (Figure 5A-B). Similarly, there were 360 DEGs between the KM-L and MOD groups, including 202 upregulated and 158 downregulated genes (Figure 5C-D). To identify the genes and signaling pathways that may be affected, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment functional analysis was conducted (Figure 5E-H). The results showed that the biological processes of DEG mainly focus on cellular processes and biological regulation, while the functions of DEG mainly focus on neuroactive ligand-receptor interactions, such as the CAMP, PPAR, PI3K-AKT, and p53 signaling pathways. These results suggest that KMF may treat MN by affecting these signaling pathways, with the PI3K/AKT signaling pathway validated in experiments. Finally, the top 100 DEGs were selected in order of degree value to construct the Protein-protein interaction (PPI) network (Figure 5I-J).
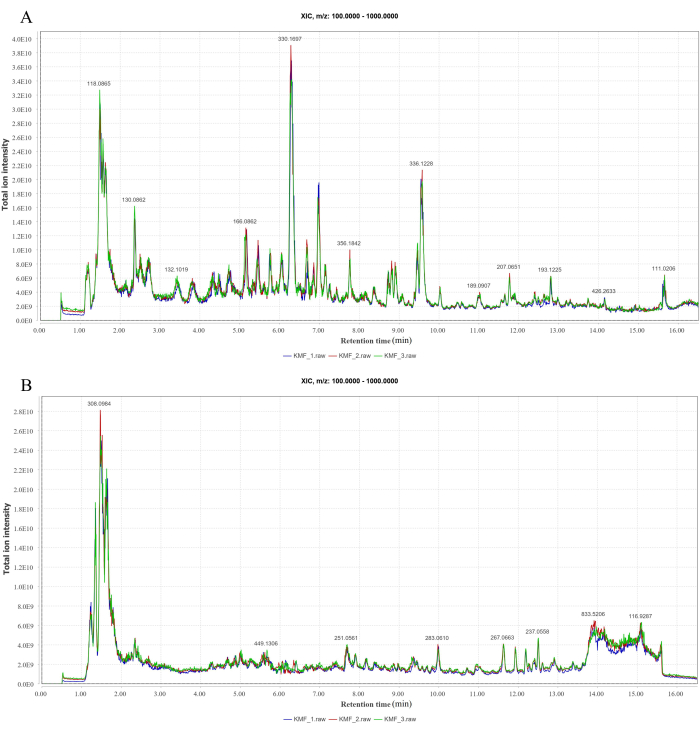
Figure 1: LC-MS/MS peak ion chromatogram. (A) Positive ion mode. (B) Negative ion mode. Please click here to view a larger version of this figure.
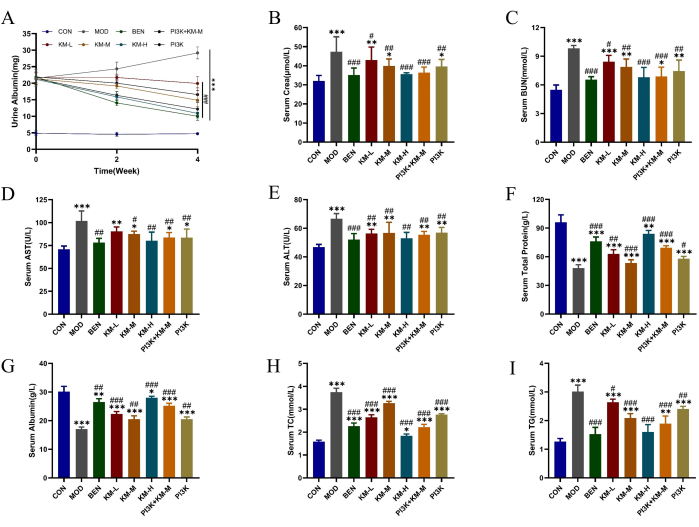
Figure 2: KMF improves lipid metabolism disorder and liver and kidney injury in MN rats. (A-C) The effects of Kemeng Fang on renal function, including 24 h urine Albumin, serum Cera, and serum BUN. (D-G) The effects of Kemeng Fang on liver function include Alanine aminotransferase (ALT), Aspartate transaminase (AST), total protein, and serum albumin. (H-I) The effect of Kemeng Fang on the regulation of blood lipid metabolism, including Triglyceride (TG) and Total cholesterol (TC). Data are expressed as means ± standard deviations of 3-6 independent samples, using one-way ANOVA in T-test, compared with the blank group, *p<0.05, **p<0.01, ***p<0.001, and compared with the model group, #p < 0.05, ##p < 0.01, ###p < 0.001. Please click here to view a larger version of this figure.
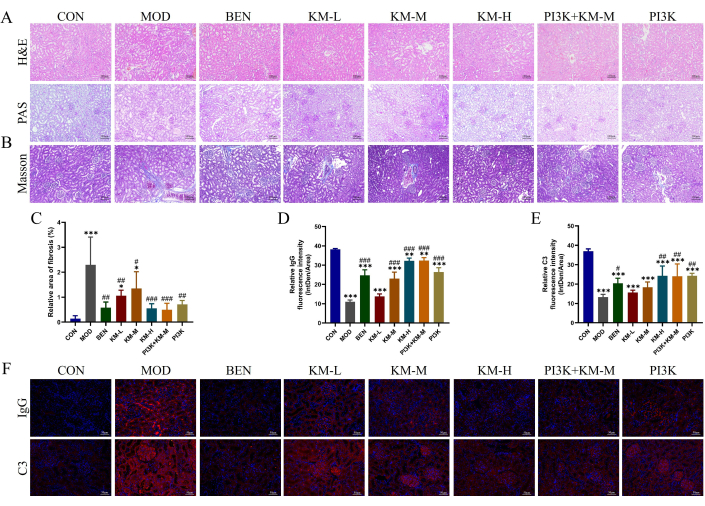
Figure 3: Kemeng Fang improves histopathological damage to the kidneys of MN rats. (A) Renal histological examination, including H&E and PAS (200x). (B-C) Semi-quantitative analysis of renal fibrosis (blue collagen fibers) relative area using Masson staining and Image J software. (D-F) Semi-quantitative analysis of relative fluorescence intensity (Intden/Area; where Intden is the total regional fluorescence intensity, the area is the regional area) of IgG and C3 in renal tissue using IF and Image J software. Please click here to view a larger version of this figure.
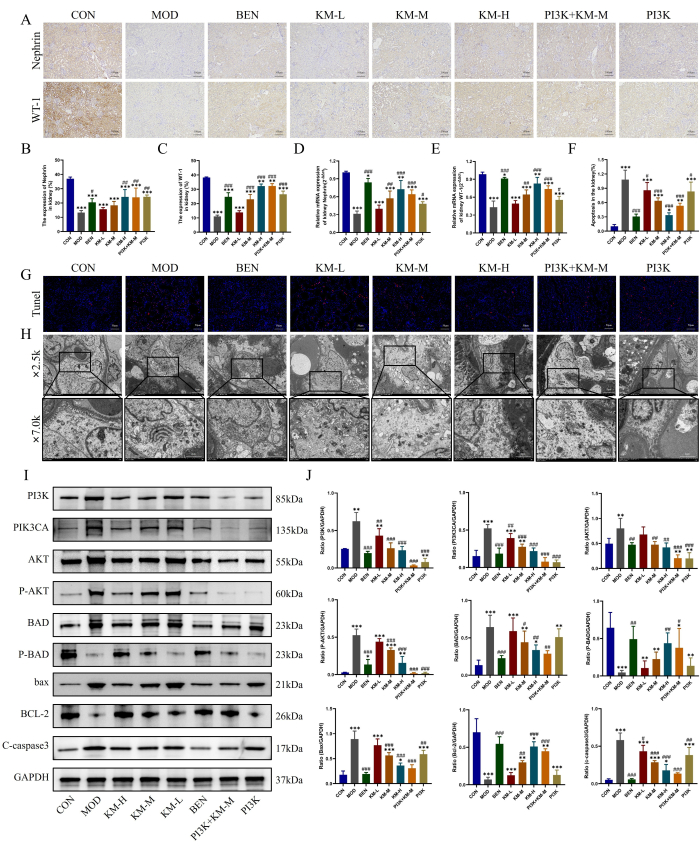
Figure 4: Kemeng Fang alleviates podocyte damage by activating the PI3K/AKT signaling pathway. (A-C) IHC was used to detect the relative expression levels of two podocyte marker proteins, WT-1, and Nephrin, in renal tissue. (D-E) PCR detection of the relative mRNA expression of two podocyte marker proteins, WT-1 and Nephrin, in renal tissue. (F-G) TUNEL staining was used to detect the incidence of apoptosis in renal tissue. (H) Observation of glomerular basement membrane and podocyte mitochondrial structure using TEM (2,500x, bar=5 µM; 7,000x, bar=2 µM). (I-J) WB detection of relative protein expression levels of PI3K, PIK3CA, AKT, P-AKT, BAD, P-BAD, BCL-2, bax, and C-caspase3 in renal tissue. Please click here to view a larger version of this figure.
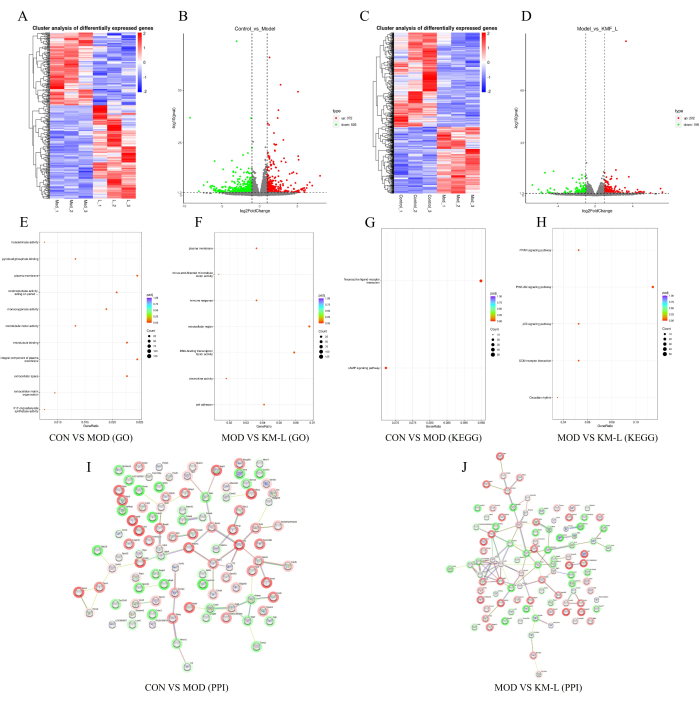
Figure 5: Exploring the possible mechanism of Kemeng Fang in treating MN based on transcriptomics. (A-B) Differential gene heatmaps and volcano plots between the CON and MOD groups, with blue representing downregulation and red representing upregulation. (C-D) Differential gene heatmaps and volcano plots between the MOD and KM-L groups, with blue representing downregulation and red representing upregulation. (E) GO enrichment between the CON and MOD groups. (F) GO enrichment between the MOD and KM-L groups. (G) KEGG enrichment between the CON and MOD groups. (H) KEGG enrichment between the MOD and KM-L groups. (I) Protein-protein interaction (PPI) chart of the top 100 differentially expressed genes between the CON and MOD groups. (J) PPI chart of the top 100 DEG degree values between the MOD and KM-L groups. Please click here to view a larger version of this figure.
| NO. | Chinese name | Latin name | Family | Part used | Dose(g) |
| 1 | Dangshen (DS) | Codonopsis pilosula (Franch.) Nannf. | Campanulaceae | Root | 20 |
| 2 | Huangqi (HQ) | Astragalus membranaceus (Fisch.) Bunge. | Leguminosae | Root | 30 |
| 3 | Huanglian (HL) | Coptis chinensis Franch. | Ranunculaceae | Root and tuber | 3 |
| 4 | Suye (SY) | Perilla frutescens (L.) Britt | Labiatae | leaf | 6 |
| 5 | Shudihuang (SDH) | Rehmannia glutinosa (Gaertn.) DC. | Scrophulariaceae | Root and tuber | 15 |
| 6 | Chuanxiong (CX) | Ligusticum chuanxiong Hort. | Umbelliferae | Root and tuber | 15 |
| 7 | Qianshi (QS) | Euryale ferox Salisb. | Nymphaeaceae | Seed | 15 |
| 8 | Qinfengteng (QFT) | Sabia japonica Maxim. | Sabiaceae | Root, tuber and leaf | 10 |
| 9 | Wubeizi (WBZ) | Rhus chinensis Mill. | Anacardiaceae | leaf | 3 |
| 10 | Banbianlian (BBL) | Lobelia chinensis Lour. | Campanulaceae | Tuber and leaf | 15 |
| 11 | Baihuasheshecao (BHSSC) | Oldenlandia diffusa (Willd.) Roxb. | Rubiaceae | Tuber and leaf | 15 |
Table 1: Composition of Kemeng Fang (KMF).
| Antibody | Dilution multiple |
| IgG | 1:100 |
| C3 | 1:100 |
| WT-1 | 1:200 |
| Nephrin | 1:100 |
| PI3K | 1:1000 |
| PI3K3CA | 1:1000 |
| AKT | 1:1000 |
| P-AKT | 1:1000 |
| BAD | 1:1000 |
| P-BAD | 1:1000 |
| BCL-2 | 1:1000 |
| bax | 1:4000 |
| c-caspase3 | 1:1000 |
| GAPDH | 1:1000 |
Table 2: Antibody dilution multiples.
| Gene | Primer | Sequence (5'-3') | PCR Products | |
| Rat GAPDH | Forward | ACAGCAACAGGGTGGTGGAC | 253 bp | |
| Reverse | TTTGAGGGTGCAGCGAACTT | |||
| Rat WT-1 | Forward | AATGGACAGAAGGGCAGAGCA | 209 bp | |
| Reverse | TGGGTACGCACACATGAAAGG | |||
| Rat Nephrin | Forward | CGGAGAACAAGAACGTGACC | 177 bp | |
| Reverse | ATTGTCTTCTCTCCGCACCA | |||
Table 3: Detailed information of qRT-PCR primers.
Supplementary Table 1: PCR reaction. Please click here to download this File.
Supplementary Table 2: Quantitative list of metabolite identification. ID: First order molecular weight serial number; Name: Identification result; Mz: mass to nucleus ratio; Rt: retention time (S); Exact Mass: Accurate molecular weight; Ppm: The error between the detected molecular weight and the theoretical molecular weight, measured in ppm; precursor_type: Ionization mode, [M+H]+ is positive ion mode, [M-H]- is negative ion mode; class: Triple classification in HMDB database; sub_class: Four level classification in HMDB database; KEGG: KEGG compound number; KEGG_Pathway: KEGG signaling pathway; CAS: Chemical Abstracts Service registration number; HMDB: HMDB database number; Library: Database; Formula: Theoretical molecular formula; KEGG: KEGG compound number; Library: Database; level: Metabolite identification level; pos: positive ion mode; neg: negative ion mode; KMF_1/2/3: total ion intensity of three experiments. Please click here to download this File.
Discussion
This study aimed to observe the pharmacological effects of KMF and explore its specific mechanism of inhibiting apoptosis of MN rat podocytes. First, it demonstrated in vivo that KMF can alleviate podocyte apoptosis and delay MN progression by activating the PI3K/AKT signaling pathway. Second, transcriptomic results showed that KMF may exert its effects through the PPAR, PI3K/AKT, and p53 signaling pathways, ECM receptor interaction, etc. Among them, the PI3K/AKT signaling pathway has been validated in experiments. These findings may provide a scientific basis for the clinical use of KMF as a potential treatment option for patients with MN.
Research has shown that podocyte apoptosis is one of the key factors leading to the gradual progression of MN. Furthermore, MN limits the division and proliferation ability of podocytes; consequently, once damaged or lost, this sequela seriously affects renal function. When the number of podocytes decreases to the point where they cannot fully cover the GBM, the GBM is completely exposed and adheres to Bowman's capsule, causing compression or even collapse of the glomerular capillary loop, ultimately promoting MN to develop into ESRD33,34,35,36. Therefore, further research on the specific mechanism of podocyte apoptosis and methods to block or inhibit podocyte apoptosis is key to delaying the progression of MN. Research has shown that the PI3K/AKT signaling pathway has multiple functions in regulating cell apoptosis, oxidative stress, and inflammatory response and plays an important regulatory role in the occurrence and development of MN. WT-1 and Nephrin are pore membrane proteins expressed on podocytes, which not only play an important role in maintaining the normal structure and function of the pore membrane but also initiate PI3K/AKT-dependent signaling pathways and participate in podocyte signaling. The decrease in expression levels of these proteins often indicates damage to podocytes37,38,39.
PI3K is a dimer composed of a regulatory subunit, p85, and a catalytic subunit, p110, which can be activated by various growth factors and complexes. It is a key and initiating factor in this pathway40. The PIK3CA gene is located on chromosome 3 and has a total of 20 exons. Its main function is to encode one of the catalytic subunits of the PI3K enzyme, p110 α Protein; hence, changes in PIK3CA can cause the PI3K enzyme to remain in a sustained activated state41. Once PI3K is activated, the second messenger, PIP3, will generate and continuously stimulate the downstream AKT signaling pathway, while p-AKT promotes the phosphorylation of the pro-apoptotic molecule, Bad, dissociating the pro-apoptotic complex of Bad with Bcl-2 and Bcl-xL, and forming a complex with the 14-3-3 protein in the cytoplasm, thereby losing its pro-apoptotic function. The anti-apoptotic molecules, BCL-2 and Bcl-xL, can be fully dissociated and exert an inhibitory effect on podocyte apoptosis42,43. Caspase-3 is a protease that plays a core role in the execution phase of cell apoptosis, ultimately leading to cell apoptosis by cleaving the DNA repair enzyme PARP into small fragments44. The induction of cytochrome c release from mitochondria to the cytoplasm by the pro-apoptotic protein Bad is a key step in activating caspase, and the complex of P-Bad binding to the 14-3-3 protein inhibits this process, thereby preventing the occurrence of the apoptotic cascade45,46,47.
Transcriptomic results indicate that KMF treatment for MN is closely related to the PI3K/AKT, PPAR, and p53 signaling pathways. Validated the PI3K/AKT signaling pathway through WB analysis. The experimental results showed that the PI3K/AKT signaling pathway in MN rats was significantly inhibited, while KMF could significantly activate the PI3K/AKT signaling pathway, reducing the generation of the pro-apoptotic molecules Bad and Bax and promoting the generation of the anti-apoptotic molecule BCL-2, thereby increasing the expression levels of podocyte hiatal membrane proteins, WT-1 and Nephrin, and reducing the incidence of podocyte apoptosis. Therefore, KMF reduces podocyte apoptosis by activating the PI3K/AKT signaling pathway and was found to have a protective effect on MN model rats.
However, this study has some limitations. First, this study only explored the mechanism of KMF inhibition of podocyte apoptosis at the level of in vivo animal experiments, which needs to be verified by in vitro cellular experiments as well as in-depth explorations of the mechanism; second, podocyte injury is also closely related to autophagy, immune-inflammation, and pyroptosis, and it needs to be further explored whether KMF can affect MN by regulating autophagy, immune-inflammation, and pyroptosis48,49.
The PPAR family (PPAR α, PPAR β/δ, PPAR γ) is a nuclear hormone receptor that relies on ligand activation and has important functions such as participating in energy metabolism, regulating cell apoptosis, and inflammatory response50,51. They affect gene transcription by forming heterodimerization with retinoic acid X receptor (RXR), where PPAR γ both regulate the inflammatory factor NF- κB. The key to B activation lies in its function of protecting podocytes from damage52,53,54. Multiple studies have shown that PPAR γ agonists (TZD, such as pioglitazone) have renal protective effects independent of hypoglycemic effects, directly protecting podocytes from damage and reducing proteinuria and glomerular damage in various kidney disease animal models55,56,57,58,59. There are also reports indicating that PPAR can reduce podocyte apoptosis by inhibiting the activation of Caspase-360. The latest research indicates a new type of PPAR γ, the selective regulator GQ-16, is more effective than TZD in reducing proteinuria and nephrotic syndrome-related complications, which also brings dawn to the treatment of kidney disease61. KMF has a protective effect on the apoptosis of MN rat podocytes by activating the PI3K/AKT signaling pathway. Based on the important role of the PPAR signaling pathway in the kidneys, further in-depth exploration of the relationship between KMF, PPAR, and MN is required in the future.
Disclosures
All the authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by the Jilin Provincial Natural Science Foundation (No.YDZJ202301ZYTS145 and No. YDZJ202301ZYTS208).
Materials
| Name | Company | Catalog Number | Comments |
| 2-Amino-3-(2-chloro-phenyl)-propionic acid | Aladdin | 103616-89-3 | |
| 30% H2O2 | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10011218 | |
| 812 embedding agent | SPI | 90529-77-4 | |
| Acetone | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10000418 | |
| Acetonitrile | Thermo | 75-05-8 | |
| Ammonia | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10002118 | |
| Ammonium formate | Sigma | 540-69-2 | |
| Analytical balance | Changzhou Lucky Electronic Equipment Co., Ltd | FA | |
| Anhydrous ethanol | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10009218 | |
| Anti fluorescence quenching and sealing agent | southernbiotech | 0100-01 | |
| Automatic biochemical analyzer | Rayto Life and Analytical Sciences | Chemray240 | |
| BCA Protein Assay Kit | Solarbio | PC0020 | |
| Benazepril hydrochloride tablets | Xinya Minhang | H20044840 | |
| Blender | Kylin-Bell | BE-2600 | |
| Brick and stone cutting blade | Daitome | Ultra45 | |
| BSA | ZSGB-BIO | ZLI-9027 | |
| Buffer RW | Beijing Baiao Leibo | WK191 | |
| Carbodiimide hydrochloride | Hubei Xinghengye Technology | 25952-53-8 | |
| Cell apoptosis detection kit | Elabscience | E-CK-A322 | |
| Chemiluminescence imaging system | Hangzhou Shenhua Technology Co., Ltd | SH-523 | |
| Chloroform | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10006818 | |
| Constant temperature drying oven | Thermo Fisher | Heto PowerDiy LL3000 | |
| Cover glass slide | Jiangsu Shitai Experimental Equipment Co., Ltd | 10212450C | |
| CY3 Conjugated AffiniPure Goat Anti-rabbit IgG (H+L) | BOSTER | BA1032 | |
| DAB reagent kit | Servicebio | G1212-200T | |
| DAPI | Blue Cloud Sky | C1002 | |
| Decolorization shaker | Wuhan Lingsi Biotechnology Co., Ltd | TSY-B | |
| Dehydration machine | Wuhan Junjie Electronics Co., Ltd | JJ-12J | |
| DL2000 DNA Marker | TIANGEN | MD114 | |
| DMSO | MCE | HY-Y0320 | |
| dNTP | TIANGEN | CD117 | |
| EBlot L1 Rapid Wet Rotation Instrument | Kingsray Biotechnology Co., Ltd | L00686C | |
| ECL substrate solution | affinity | KF8003 | |
| Electric constant temperature water bath pot | Fisaff Instrument (Hebei) Co., Ltd | DK-20000-IIIL | |
| Electrophoresis instrument power supply | Beijing Longfang Technology Co., Ltd | LF-600S | |
| Embedding machine | Wuhan Junjie Electronics Co., Ltd | JB-P5 | |
| Equilibrium buffer | Wuhan Lingsi Biotechnology Co., Ltd | E8090 | |
| Ethylenediamine | Ruichengkang Pharmaceutical Technology | 107-15-3 | |
| FA series multifunctional analytical electronic balance | Changzhou Lucky Electronic Equipment Game Company | FA1204 | |
| Filter membrane | Jinteng | Nylon6-0.22μm | |
| Formaldehyde solution | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10010018 | |
| Formic acid | TCI | 64-18-6 | |
| Freezing centrifuge | Xiangyi | H1850-R | |
| Frozen platform | Wuhan Junjie Electronics Co., Ltd | JB-L5 | |
| Glacial acetic acid | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10000218 | |
| Glass bead | Sigma | G8772-500G | |
| Glass slide | Nantong Meiweide Life Science Co., Ltd | PC2-301 | |
| glycine | Biofroxx | 30166428 | |
| Hematoxylin | Wuhan Lingsi Biotechnology Co., Ltd | G1140 | |
| High speed refrigerated centrifuge | Hunan Kecheng Instrument Equipment Co., Ltd | H1-16KR | |
| Horizontal agarose electrophoresis tank | Long Fang | LF-31DS | |
| Horizontal shaker | Jiangsu Haimen Qilin Bell Instrument Manufacturing Co., Ltd | TS-1 | |
| HRP Conjugated AffiniPure Goat Anti-rabbit IgG (H+L) | BOSTER | BA1054 | |
| hydrochloric acid | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10011028 | |
| Imaging system | Nikon | Nikon DS-U3 | |
| Incomplete Freund's adjuvant | MCE | ISA-51 | |
| Intelligent digital magnetic heating stirrer | Hangzhou Miou Instrument Co., Ltd | TP-350E+ | |
| Isoflurane | Sigma | 26675-46-7 | |
| Isopropanol | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 80109218 | |
| KCl | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10020318 | |
| KH2PO4 | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 1115GR500 | |
| Liquid chromatograph | Thermo | IQLAAAGABHFAPUMBJC | |
| marker(10-250KD) | Mei5bio | MF028-plus-01 | |
| marker(20-120KD) | GenScript | M00521 | |
| Mass spectrometer | Thermo | IQLAAEGAAPFALGMBDK | |
| Masson staining kit | BASO | BA4079B | |
| Methanol | Thermo | 67-56-1 | |
| microscope | Nikon | ECLIPSE Ci | |
| microwave oven | Galanz Microwave Oven Electrical Appliance Co., Ltd | P70D20TL-P4 | |
| Multi sample tissue grinder | Shanghai Jingxin Industrial Development Co., Ltd | Tissuelyser-24L | |
| Multiskan FC ELISA reader | Thermo scientific | 1410101 | |
| Na2HPO4.12H2O | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10017618 | |
| NaCl | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10000218 | |
| Neutral resin | Wuhan Lingsi Biotechnology Co., Ltd | G8590 | |
| Normal Goat Serum | Solarbio | SL038 | |
| Organizational spreading machine | Zhejiang Jinhua Kedi Instrument Equipment Co., Ltd | KD-P | |
| Osmic acid | Ted Pella Inc | 18450 | |
| oven | Shanghai Huitai Instrument Manufacturing Co., Ltd | DHG-9140A | |
| Palm centrifuge | Wuhan Lingsi Biotechnology Co., Ltd | D1008E | |
| Paraformaldehyde | Solarbio | P1110 | |
| PAS staining kit | BASO | BA4114B | |
| Pathological slicer | Shanghai Leica Instrument Co., Ltd | RM2016 | |
| PBS | Solarbio | P1020 | |
| PCR instrument | Hangzhou Miou Instrument Co., Ltd | PR-96 | |
| PH meter | Sedolis Scientific Instruments (Beijing) Co., Ltd | PB-10 | |
| PH meter | Sedolis Scientific Instruments (Beijing) Co., Ltd | 2018C132-11 | |
| PI3K inhibitor LY294002 | MCE | HY-10108 | |
| Pipette gun | Dragon | KE0003087/KA0056573 | |
| Protein phosphatase inhibitor complex | Meilunbio | MB12707-1 | |
| PVDF membrane (0.22 μm) | Solarbio | ISEQ00010 | |
| PVDF membrane (0.45 μm) | Solarbio | YA1701 | |
| Quick primary/secondary antibody diluent | Solarbio | A1811 | |
| Rabbit anti-AKT | Affinity | AF0836 | |
| Rabbit anti-BAD | Affinity | AF6471 | |
| Rabbit anti-C3 | Affinity | DF13224 | |
| Rabbit anti-GAPDH | Hangzhou Xianzhi | AB-P-R 001 | |
| Rabbit anti-IgG | CST | 3900S | |
| Rabbit anti-Nephrin | bioss | bs-10233R | |
| Rabbit anti-P-AKT | Affinity | AF0016 | |
| Rabbit anti-p-BAD | invitrogen | PA5-105023 | |
| Rabbit anti-PI3K | Affinity | AF6241 | |
| Rabbit anti-PIK3CA | Bioss | Bs-2067R | |
| Rabbit anti-WT-1 | Affinity | DF6331 | |
| Real-Time PCR System | ABI | QuantStudio 6 | |
| RIPA Lysis Buffer | Meilunbio | MA0151 | |
| SDS | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10019318 | |
| Slide and cover glass | Jiangsu Shitai Experimental Equipment Co., Ltd | 10212432C | |
| Super pure water instrument | Zhiang Instrument (Shanghai) Co., Ltd | Clever-S15 | |
| SYBR Green Master Mix | VAZYME | Q111-02 | |
| Taq Plus DNA Polymerase | TIANGEN | ET105-02 | |
| Tissue grinder | Beautiful Wall | MB-96 | |
| Transmission electron microscope | HITACHI | HT7800/HT7700 | |
| Tris-base | Biofroxx | 10019318 | |
| Trizol | Ambion | 15596-026 | |
| TUNEL Cell Apoptosis Detection Kit (FITC) | Beijing Baiao Leibo | SY0475 | |
| Tween 20 | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 30189328 | |
| Ultra micro UV visible spectrophotometer | Hangzhou Miou Instrument Co., Ltd | ND-100 | |
| Ultra thin slicer | Leica | Leica UC7 | |
| Ultrasonic cleaner | shumei | KQ- 800DE | |
| Upright optical microscope | Nikon | Nikon Eclipse CI | |
| Urinary Protein Test Kit | Nanjing Jiancheng Bioengineering Research Institute | C035-2 | |
| Vertical electrophoresis tank | Beijing 61 Instrument Factory | DYCZ-24DN | |
| Vortex mixer | Wuhan Lingsi Biotechnology Co., Ltd | MX-F | |
| Western Blocking Buffer | Solarbio | SW3010 | |
| xylene | China National Pharmaceutical Group Chemical Reagent Co., Ltd | 10023418 |
References
- McQuarrie, E. P., Mackinnon, B., Stewart, G. A., Geddes, C. C., Registry, S. R. B. Membranous nephropathy remains the commonest primary cause of nephrotic syndrome in a northern European Caucasian population. Nephrol Dial Transplant. 25 (3), 1009-1011 (2010).
- Ronco, P., Debiec, H. Pathophysiological advances in membranous nephropathy: time for a shift in patient's care. Lancet. 385 (9981), 1983-1992 (2015).
- Xu, X., et al. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol. 27 (12), 3739-3746 (2016).
- Fogo, A. B., Lusco, M. A., Najafian, B., Alpers, C. E. AJKD atlas of renal pathology: Membranous nephropathy. Am J Kidney Dis. 66 (3), e15-e17 (2015).
- Li, X., Zhang, X. Comments on the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for anticoagulant therapy in patients with membranous nephropathy. Kidney Int. 101 (1), 186-187 (2022).
- Ruggenenti, P., Fervenza, F. C., Remuzzi, G. Treatment of membranous nephropathy: time for a paradigm shift. Nat Rev Nephrol. 13 (9), 563-579 (2017).
- Klomjit, N., Fervenza, F. C., Zand, L. Successful treatment of patients with refractory PLA2R-associated membranous nephropathy with Obinutuzumab: A report of 3 cases. Am J Kidney Dis. 76 (6), 883-888 (2020).
- Podestà, M. A., Ruggiero, B., Remuzzi, G., Ruggenenti, P. Ofatumumab for multirelapsing membranous nephropathy complicated by rituximab-induced serum-sickness. BMJ Case Rep. 13 (1), e232890-e232896 (2020).
- Stehlé, T., et al. Anti-CD38 therapy for PLA2R-positive membranous nephropathy resistant to conventional immunosuppression. Kidney Int. 101 (2), 416-418 (2022).
- Trimarchi, H., Coppo, R. Podocytopathy in the mesangial proliferative immunoglobulin A nephropathy: new insights into the mechanisms of damage and progression. Nephrol Dial Transplant. 34 (8), 1280-1285 (2019).
- Kopp, J. B., et al. Podocytopathies. Nat Rev Dis Primers. 6 (1), 68-72 (2020).
- Hoxha, E., Reinhard, L., Stahl, R. A. K. Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev Nephrol. 18 (7), 466-478 (2022).
- Chiou, T. T., et al. Rapamycin attenuates PLA2R activation-mediated podocyte apoptosis via the PI3K/AKT/mTOR pathway. Biomed Pharmacother. 144 (8), 112349-112356 (2021).
- Chen, J., et al. Danshen injection induces autophagy in podocytes to alleviate nephrotic syndrome via the PI3K/AKT/mTOR pathway. Phytomedicine. 107 (7), 154477-154489 (2022).
- Wang, Y., et al. Fangji Huangqi decoction ameliorates membranous nephropathy through the upregulation of BNIP3-mediated mitophagy. J Ethnopharmacol. 324 (13), 117734-117743 (2024).
- Wei, L., et al. Shenqi granule upregulates CD2AP and α-actinin4 and activates autophagy through regulation of mTOR/ULK1 pathway in MPC5 cells. J Ethnopharmacol. 303 (16), 115942-115954 (2023).
- Wang, P., et al. A review of the botany, phytochemistry, traditional uses, pharmacology, toxicology, and quality control of the Astragalus memeranaceus. Front Pharmacol. 14 (2), 1242318-1242325 (2023).
- Wang, Q., et al. Traditional Chinese medicine method of tonifying kidney for hypertension: Clinical evidence and molecular mechanisms. Front Cardiovasc Med. 20229 (16), 1038480-1038487 (2022).
- Tang, X., et al. Experimental models for elderly patients with membranous nephropathy: Application and advancements. Exp Gerontol. 185 (8), 112341-112353 (2024).
- Zhang, J. J., et al. Murine membranous nephropathy: immunization with α3(IV) collagen fragment induces subepithelial immune complexes and FcγR-independent nephrotic syndrome. J Immunol. 188 (7), 3268-3277 (2012).
- Hopfer, H., et al. The importance of cell-mediated immunity in the course and severity of autoimmune anti-glomerular basement membrane disease in mice. FASEB J. 17 (8), 860-868 (2003).
- Fleuren, G., Grond, J., Hoedemaeker, P. J. In situ formation of subepithelial glomerular immune complexes in passive serum sickness. Kidney Int. 17 (5), 631-637 (1980).
- Border, W. A., Ward, H. J., Kamil, E. S., Cohen, A. H. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J Clin Invest. 69 (2), 451-461 (1982).
- Border, W. A., Ward, H. J., Kamil, E. S., Cohen, A. H. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J Clin Invest. 69 (2), 451-461 (1982).
- Rubio-Navarro, A., et al. Phenotypic characterization of macrophages from rat kidney by flow cytometry. J Vis Exp. (116), e54599 (2016).
- Hou, Y., et al. Longzhibu disease and its therapeutic effects by traditional Tibetan medicine: Ershi-wei Chenxiang pills. J Ethnopharmacol. 249, 112426 (2020).
- Sethi, S., et al. Hematopoietic stem cell transplant-membranous nephropathy is associated with protocadherin FAT1. J Am Soc Nephrol. 33 (5), 1033-1044 (2022).
- Gong, Q., Lai, T., Liang, L., Jiang, Y., Liu, F. Targeted inhibition of CX3CL1 limits podocytes ferroptosis to ameliorate cisplatin-induced acute kidney injury. Mol Med. 29 (1), 140-148 (2023).
- Li, H., et al. IL-23 reshapes kidney resident cell metabolism and promotes local kidney inflammation. J Clin Invest. 131 (12), e142428-e142441 (2021).
- Wang, C., et al. Artificially cultivated Ophiocordyceps sinensis alleviates diabetic nephropathy and its podocyte injury via inhibiting P2X7R expression and NLRP3 inflammasome activation. J Diabetes Res. 356 (11), 1390418-1390431 (2018).
- Cheng, H., et al. PI3K/Akt signaling pathway and Hsp70 activate in hippocampus of rats with chronic manganese sulfate exposure. J Trace Elem Med Biol. 50 (12), 332-338 (2018).
- Ji, K., et al. The immunoreaction and antioxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala) involves the PI3K/Akt/Nrf2 and NF-κB signal pathways in response to dietary methionine levels. Fish Shellfish Immunol. 105 (10), 126-134 (2020).
- Chiou, T. T., et al. Rapamycin attenuates PLA2R activation-mediated podocyte apoptosis via the PI3K/AKT/mTOR pathway. Biomed Pharmacother. 144 (3), 112349-112357 (2021).
- Lv, Q., Yang, F., Chen, K., Zhang, Y. Autophagy protects podocytes from sublytic complement induced injury. Exp Cell Res. 341 (2), 132-138 (2016).
- Mundel, P., Shankland, S. J. Podocyte biology and response to injury. J Am Soc Nephrol. 13 (12), 3005-3015 (2002).
- Sun, Z., Xu, Q., Ma, Y., Yang, S., Shi, J. Circ_0000524/miR-500a-5p/CXCL16 axis promotes podocyte apoptosis in membranous nephropathy. Eur J Clin Invest. 51 (3), e13414-e13422 (2021).
- Huber, T. B., et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 23 (14), 4917-4928 (2003).
- Ai, Z., et al. Deciphering the pharmacological mechanisms of Rostellularia procumbens (L) Nees. Extract alleviates adriamycin-induced nephropathy in vivo and in vitro. Phytomedicine. 113 (6), 154736-154742 (2023).
- Wang, X., et al. Nicorandil alleviates apoptosis in diabetic cardiomyopathy through PI3K/Akt pathway. J Cell Mol Med. 23 (8), 5349-5359 (2019).
- Fox, M., Mott, H. R., Owen, D. Class IA PI3K regulatory subunits: p110-independent roles and structures. Biochem Soc Trans. 48 (4), 1397-1417 (2020).
- Miricescu, D., et al. PI3K/AKT/mTOR signaling pathway in breast cancer: From molecular landscape to clinical aspects. Int J Mol Sci. 22 (1), 173-179 (2020).
- Feng, C., et al. Neuroprotective effect of Danhong injection on cerebral ischemia-reperfusion injury in rats by activation of the PI3K-Akt pathway. Front Pharmacol. 11 (4), 298-312 (2020).
- Zhou, Y., et al. 1,3-Dicaffeoylquinic acid targeting 14-3-3 tau suppresses human breast cancer cell proliferation and metastasis through IL6/JAK2/PI3K pathway. Biochem Pharmacol. 172 (12), 113752-113761 (2020).
- Li, J., et al. Tetrandrine inhibits colon carcinoma HT-29 cells growth via the Bcl-2/Caspase 3/PARP pathway and G1/S phase. Biosci Rep. 39 (5), (2019).
- Downward, J. How BAD phosphorylation is good for survival. Nat Cell Biol. 1 (2), E33-E35 (1999).
- Fan, J., et al. A model of ischemia and reperfusion increases JNK activity, inhibits the association of BAD and 14-3-3, and induces apoptosis of rabbit spinal neurocytes. Neurosci Lett. 473 (3), 196-201 (2010).
- Chipuk, J. E., Green, D. R. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization. Trends Cell Biol. 18 (4), 157-164 (2008).
- Yin, L., Yu, L., He, J. C., Chen, A. Controversies in podocyte loss: Death or detachment. Front Cell Dev Biol. 9 (11), 771931-771942 (2021).
- Yang, C., et al. Research progress on multiple cell death pathways of podocytes in diabetic kidney disease. Mol Med. 29 (1), 135-143 (2023).
- Calvier, L., et al. PPARγ links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell Metab. 25 (5), 1118-1134 (2017).
- Dai, Z. W., Cai, K. D., Xu, L. C., Wang, L. L. Perilipin2 inhibits diabetic nephropathy-induced podocyte apoptosis by activating the PPARγ signaling pathway. Mol Cell Probes. 53 (3), 101584-101596 (2020).
- Kota, B. P., Huang, T. H., Roufogalis, B. D. An overview on biological mechanisms of PPARs. Pharmacol Res. 51 (2), 85-94 (2005).
- Wahli, W. Peroxisome proliferator-activated receptors (PPARs): from metabolic control to epidermal wound healing. Swiss Med Wkly. 132 (7-8), 83-91 (2002).
- Agrawal, S., He, J. C., Tharaux, P. L. Nuclear receptors in podocyte biology and glomerular disease. Nat Rev Nephrol. 17 (3), 185-204 (2021).
- Miglio, G., et al. Protective effects of peroxisome proliferator-activated receptor agonists on human podocytes: proposed mechanisms of action. Br J Pharmacol. 167 (3), 641-653 (2012).
- Miglio, G., et al. The subtypes of peroxisome proliferator-activated receptors expressed by human podocytes and their role in decreasing podocyte injury. Br J Pharmacol. 162 (1), 111-125 (2011).
- Agrawal, S., Guess, A. J., Benndorf, R., Smoyer, W. E. Comparison of direct action of thiazolidinediones and glucocorticoids on renal podocytes: protection from injury and molecular effects. Mol Pharmacol. 80 (3), 389-399 (2011).
- Sonneveld, R., et al. Sildenafil prevents podocyte injury via PPAR-γ-mediated TRPC6 inhibition. J Am Soc Nephrol. 28 (5), 1491-1505 (2017).
- Agrawal, S., et al. Pioglitazone enhances the beneficial effects of glucocorticoids in experimental nephrotic syndrome. Sci Rep. 6 (3), 24392-24404 (2016).
- Kanjanabuch, T., et al. PPAR-gamma agonist protects podocytes from injury. Kidney Int. 71 (12), 1232-1239 (2007).
- Bryant, C., et al. Selective modulator of nuclear receptor PPARγ with reduced adipogenic potential ameliorates experimental nephrotic syndrome. iScience. 25 (4), 104001 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved