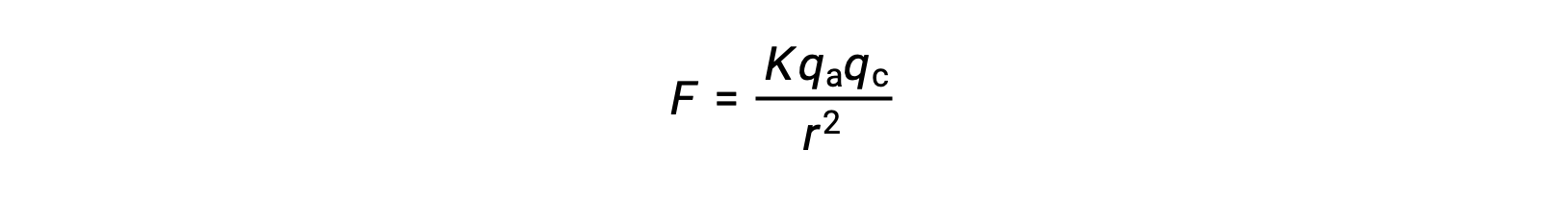Molecular solids are a type of crystalline solids which have molecules or atoms as their constituent particles and are held together by non-ionic intermolecular forces like hydrogen bonds, dispersion forces, or dipole–dipole interactions.
The strength of these intermolecular forces dictates the properties of molecular solids. Overall, these solids tend to be soft, have low melting points, and have low thermal and electrical conductivity.
Nonpolar or net nonpolar molecular solids like solid nitrogen or dry ice are primarily held together by weak dispersion forces. Such solids have very low melting points and sublime easily.
Polar molecular solids like ice and solid sulfur dioxide exhibit hydrogen bonds and dipole–dipole interactions. Such solids have comparatively higher melting points and exist as soft solids or volatile liquids at standard temperature and pressure.
Another example of how the strength of the intermolecular forces influences properties of molecular solids is illustrated by solid iodine.
The increased strength of certain intermolecular forces between larger molecules is reflected in iodine’s properties. Although they are both nonpolar solids, iodine’s melting point is substantially higher than that of solid nitrogen.
Ionic solids are crystalline solids with electrically charged species or ions as constituent particles held together by strong electrostatic forces. For example, sodium chloride is an ionic solid composed of sodium cations and chloride anions.
The packing of ionic solids maximizes the interaction between oppositely charged ions and minimizes the interaction between ions of the same charge. This is often visualized as one set of ions on lattice points and the opposing ions occupying some or all of the spaces between them, or the interstitial sites.
Due to strong coulombic interactions, ionic solids have high melting temperatures. The ionic interactions typically become stronger with an increase in charge or a decrease in ion size.
For example, caesium chloride melts at 645 °C and sodium chloride melts at 801 °C, which can be attributed to the smaller sodium cation enabling closer packing. Calcium oxide, which has higher ionic charges, melts at 2572 °C.