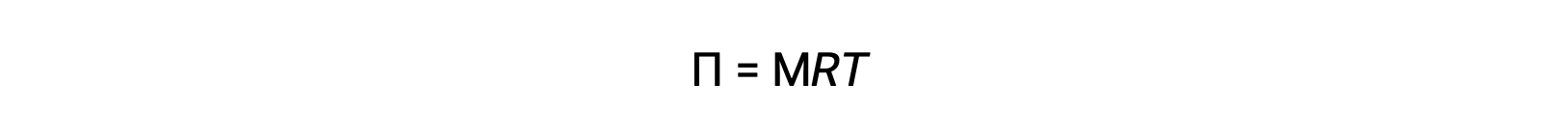A semipermeable membrane allows some substances to pass through but not others. This movement of solvent molecules across a semipermeable membrane, to a solution with higher solute concentration, is called osmosis.
Consider a U-shaped tube containing pure water on the left and a sugar solution on the right separated by a semipermeable membrane.
Water molecules will flow to the sugar solution at a faster rate than the reverse to try to establish a concentration equilibrium.
As water flows to the right, the level of liquid in the two arms becomes unequal.
Eventually, the added weight of the excess water on the right creates sufficient pressure to stop osmosis.
The minimum pressure required to halt osmosis is called the osmotic pressure. It is a colligative property that is dependent on the solute concentration in the solution.
As the concentration of the solute increases, the osmotic pressure increases proportionally.
Osmotic pressure, π, can be calculated by multiplying the molarity of the solute by the temperature in kelvin and the ideal gas constant R, 0.0821 liter-atmosphere per mole kelvin.
If the concentration of the sugar solution is 1.00 molar, then at 25 °C or 298 K, the osmotic pressure will be 24.5 atmospheres.
If the osmotic pressure of the two solutions is equal, they are called isotonic.
If one solution has a lower osmotic pressure, then it is hypotonic compared to the solution with higher solute concentration.
The concentrated solution is called hypertonic compared to the dilute solution.
When red blood cells are placed in a hypertonic solution, water leaves through the pores of the semi-permeable cell membrane. This process is called crenation and it causes the cells to become shriveled.
Conversely, if the red blood cells are placed in a hypotonic solution, water moves from the outside into the cells causing the cells to swell and ultimately rupture in a process called hemolysis.
When a person is given intravenous fluids, the fluids must be isotonic with the intracellular solution of blood cells to prevent crenation or hemolysis.