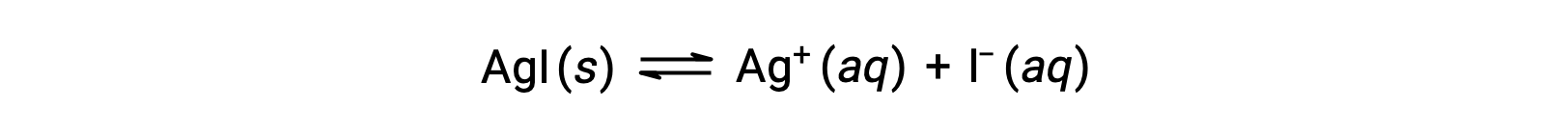Acetic acid, a weak acid, partially dissociates in solution to produce hydronium and acetate ions, while its salt, sodium acetate, dissociates completely to produce sodium ions and acetate. Both acetic acid and sodium acetate have the acetate ion in common.
When sodium acetate is added to an acetic acid solution, it increases the total concentration of acetate ions and disturbs the equilibrium. To counterbalance that change, the equilibrium shifts to the left and causes production of acetic acid until the equilibrium is reestablished.
In this case, the presence of the common ion results in the decreased dissociation of a compound. This phenomenon is known as the common ion effect.
The common ion effect can be explained with the help of Le Châtelier’s principle, which states that a change in the concentration of the reactants or products at equilibrium will cause the system to shift in a direction that counterbalances the change.
The pH of a 0.050 molar ammonia solution is 10.97. If 0.040 molar ammonium chloride is added into the solution, the new pH can be determined using the base dissociation constant of ammonia and an ICE table.
Ammonium chloride ionizes completely to produce 0.040 molar of both ammonium and chloride ions. As chloride ions are pH-neutral, they can be ignored.
Ammonia partially dissociates to produce ammonium and hydroxide ions. The Kb for this reaction is 1.76 × 10−5 and is equal to the concentration of ammonium times the concentration of hydroxide divided by the concentration of the ammonia.
The values for the initial, change, and equilibrium concentrations are placed in the ICE table, with changes in the concentration denoted by x.
Due to the small value of x, 0.050 minus x is approximately equal to 0.050, and 0.040 plus x is approximately equal to 0.040, which can be verified later by the 5% rule.
Substituting these values into the expression for Kb, x equals 2.2 × 10−5 molar.
The approximation is valid as the hydroxide concentration is less than 5% of 0.040 molar.
The pOH and pH of the solution can be calculated using the standard equations and equal 4.66 and 9.34, respectively.
Therefore, the presence of the common ion, ammonium ion, causes decreased dissociation of ammonia and thereby reduces the pH of the solution from 10.97 to 9.34.