このコンテンツを視聴するには、JoVE 購読が必要です。 サインイン又は無料トライアルを申し込む。
Measuring SMC Contraction In Vitro: A Non-Invasive Method to Evaluate Smooth Muscle Cells Contraction using Electric Cell-Substrate Impedance Sensing
Overview
This video describes the measurement of aortic smooth muscle cell (SMC) contraction using electrical cell-substrate impedance sensing, or ECIS. ECIS measures the locomotion of cells and is a reliable method for measuring contractions.
プロトコル
All procedures involving human participants have been performed in compliance with the institutional, national, and international guidelines for human welfare and have been reviewed by the local institutional review board
1. Measuring contraction (example SMCs)
- Prepare a 96w10e ECIS array (see Figure 1A for a representative image of the array with magnified electrodes and cells seeded on the electrodes).
NOTE: Perform the following steps under a sterile tissue culture laminar flow hood.- Coat a sterile 96w10e array with 200 µL of 10 mM L-cysteine per well for 30 min at room temperature.
- Wash the plate twice with sterile water. Let the plate dry overnight in the tissue culture hood with the lid slightly open.
NOTE: Coating the plate with L-cysteine is only necessary when using the plate for the first time. - The next day, UV-sterilize the plate and lid for 30 min. Turn the lid upwards so that the inside is sterilized. Once the plate is sterilized, do not open the plate outside the flow hood.
- Seeding cells on the ECIS array
- Prewarm 1% sterile gelatin solution in the water bath for 30 min.
NOTE: The solution is stored in the fridge and might solidify, making it difficult to pipette. - Subsequently, coat the wells with 100 µL of the 1 % gelatin solution per well and incubate the plate at 37 °C for at least 1 h.
- Aspirate the gelatin from the wells.
- Count the cells using an automated cell counter and seed the SMCs in triplicate at a seeding density of 30,000 cells/well in 200 µL of SMC medium.
- Place the plate with the SMCs into the ECIS 96-well holder in the cell culture incubator. Double-click on the ECIS Applied Biophysics software to open the program and press the Setup button.
- Check if all the electrodes have contact with the holder (green; red if no connection) in the left lower panel labeled Well Configuration. If the electrodes are not properly connected, adjust the plate in the holder before starting the measurement.
- Select the plate type (96-well array) in the same panel by clicking Array type.
- In the right upper panel Data Collection Setup, click on single frequency and change the impedance value to 4000 Hz and the interval to 8 s.
- Click the Start button to start measurements. Wait for a new panel to open, where the ECIS file can be saved.
- Allow the cells to attach and establish a monolayer for 48 h.
NOTE: The attachment and spreading of cells on the electrodes generate a baseline resistance value (Figure 1B).
- Prewarm 1% sterile gelatin solution in the water bath for 30 min.
- Stimulation of cells to induce contraction
- Induce SMC contraction by stimulating the cells with 10 µg/mL of the calcium ionophore, ionomycin.
NOTE: Ionomycin will induce the influx of extracellular Ca2+, activating the contractile cascade; see Figure 2A for representative images of non-stimulated and stimulated contracted cells. - Dilute 1 mg of ionomycin powder in 100 µL of dimethylsulfoxide to make a stock solution of 10 mg/mL, and store 10 µL aliquots at -20 °C. Before use, dilute the ionomycin solution 1:10 in the SMC medium to prepare the working solution to be added to the cells.
- Perform the cell stimulation while the array is still in the array holder inside the cell culture incubator and the electrodes are attached to the system.
- To stimulate the cells, remove the lid and place it on a sterile surface inside the incubator. Prepare two pipettes, set at 2 µL and 150 µL.
- Before starting the stimulation, press Mark in the software and place a comment.
NOTE: This will make it easier to find the exact timing of the stimulation when analyzing the data. - Pipette 2 µL of the ionomycin working solution into each well as quickly as possible. Once all the cells have been stimulated, mix the medium in the wells using the second pipette.
NOTE: Due to the rapid effects, it is not necessary to change tips between different cell lines and conditions. Work quickly while stimulating and mixing because the ionomycin has an acute effect. When stimulating a full plate, stimulate a maximum of 3 columns at once. After every stimulation, wait at least 30 min until the next stimulation to let the cells recover from the temperature and CO2 change caused by the opening of the incubator door. - Directly after the stimulation is done, press Mark again to add a comment that the stimulation is done.
- Induce SMC contraction by stimulating the cells with 10 µg/mL of the calcium ionophore, ionomycin.
- Finishing the stimulation experiment
- Record the impedance values for approximately 5 min after the ionomycin stimulation. Finish the recording by pressing Finish.
結果
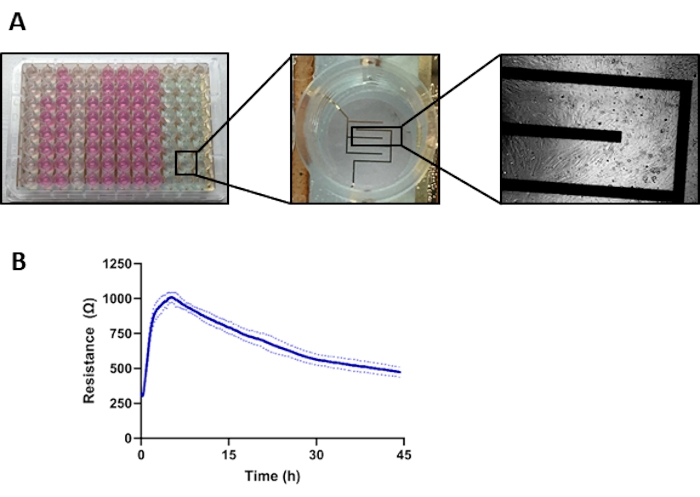
Figure 1: ECIS plate setup and graphic representation of aortic SMC contraction. (A) Left; a 96-well ECIS plate. Middle; magnification of one well of the ECIS plate, showing the ten electrodes at the bottom of the well. Right; light microscope image of the SMCs seeded on the ECIS plate; magnification 10x. (B) Resistance curves measured by ECIS after seeding of co...
開示事項
資料
| Name | Company | Catalog Number | Comments |
| 96-well Array | Applied Biophysics | 96W10idf PET | Array used to measure contraction in the ECIS setup |
| Human Vascular Smooth Muscle Cell Basal Medium (formerly ''Medium 231'') | Gibco | M231500 | Medium used to culture smooth muscle cells |
| Dimethyl sulfoxide | Sigma-Aldrich | 472301 | Solution used to dilute ionomycin |
| Invitrogen countess II | Thermo Fisher Scientific | AMQAX1000 | Automated cell counter |
| Ionomycin calcium salt from Streptomyces conglobatus | Sigma-Aldrich | I0634-1MG | Compound used for contraction stimulation |
| ZTheta | Applied Biophysics | ZTheta | ECIS instrument used for contraction measurements |
参考文献
This article has been published
Video Coming Soon
Source: Bogunovic, N. et al., Isolation of Primary Patient-specific Aortic Smooth Muscle Cells and Semiquantitative Real-time Contraction Measurements In Vitro. J. Vis. Exp. (2022).
Copyright © 2023 MyJoVE Corporation. All rights reserved