4.8 : Chemical Reactions in Aqueous Solutions
Chemical substances interact in many different ways. Certain chemical reactions exhibit common patterns of reactivity. Due to the vast number of chemical reactions, it becomes necessary to classify them based on the observed patterns of interaction.
Water is a good solvent that can dissolve many substances. For this reason, many chemical reactions take place in water. Such reactions are called aqueous reactions. The three most common types of aqueous reactions are precipitation, acid-base, and oxidation-reduction.
Reactions in Aqueous Solutions
A precipitation reaction involves the exchange of ions between ionic compounds in aqueous solution to form an insoluble salt or a precipitate. In an acid-base reaction, an acid reacts with a base, and the two neutralize each other, producing salt and water. An oxidation–reduction reaction involves the transfer of electrons between reacting species. The reactant that loses electrons is said to be oxidized, and the reactant that gains electrons is said to be reduced.
Equations for Aqueous Reactions
When ions are involved, there are various ways of representing the reactions that take place in aqueous media, each with a different level of detail. To understand this, let us take an example of a precipitation reaction. The reaction is between aqueous solutions of ionic compounds, like BaCl2 and AgNO3. The products of the reaction are aqueous Ba(NO3)2 and solid AgCl.

This balanced equation is called a molecular equation. Molecular equations provide stoichiometric information to make quantitative calculations and also helps identify the reagents used and the products formed. However, molecular equations do not provide the details of the reaction process in solution; that is, it does not indicate the different ionic species that are present in solution.
Ionic compounds such as BaCl2, AgNO3, and Ba(NO3)2 are water-soluble. They dissolve by dissociating into their constituent ions, and their ions are homogeneously dispersed in solution.



Since AgCl is an insoluble salt, it does not dissociate into ions and stays in solution as a solid. Considering the above factors, a more realistic representation of the reaction would be:
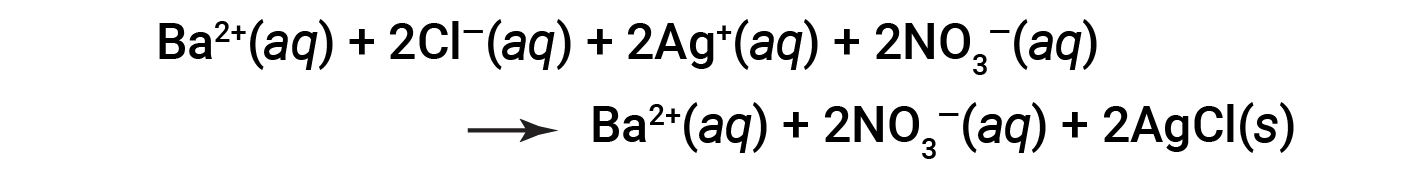
This is the complete ionic equation in which all dissolved ions are explicitly represented.
This complete ionic equation indicates two chemical species that are present in identical form on both sides, Ba2+ (aq) and NO3− (aq). These are called spectator ions. The presence of these ions is required to maintain charge neutrality. Since they are neither chemically nor physically changed by the process, they may be eliminated from the equation.

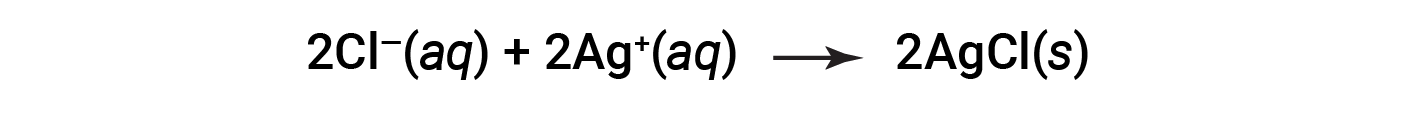
This equation can be further simplified to give:
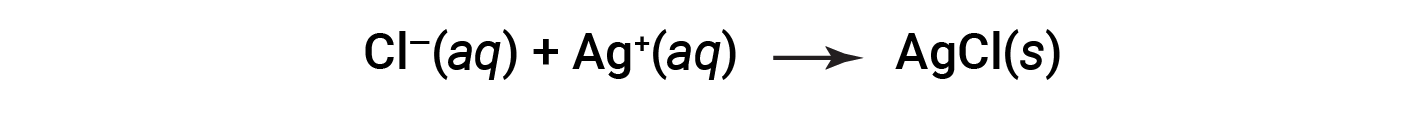
This is the net ionic equation. It indicates that solid silver chloride may be produced from dissolved chloride and silver ions, regardless of the source of these ions.
This text is adapted from OpenStax Chemistry 2e, Section 4.1: Writing and Balancing Chemical Equations.
Z rozdziału 4:

Now Playing
4.8 : Chemical Reactions in Aqueous Solutions
Chemical Quantities and Aqueous Reactions
59.5K Wyświetleń

4.1 : Stechiometria reakcji
Chemical Quantities and Aqueous Reactions
64.1K Wyświetleń

4.2 : Ograniczanie reagenta
Chemical Quantities and Aqueous Reactions
57.1K Wyświetleń

4.3 : Wydajność reakcji
Chemical Quantities and Aqueous Reactions
48.9K Wyświetleń

4.4 : Ogólne właściwości rozwiązań
Chemical Quantities and Aqueous Reactions
30.0K Wyświetleń

4.5 : Zatężanie i rozcieńczanie roztworu
Chemical Quantities and Aqueous Reactions
83.3K Wyświetleń

4.6 : Roztwory elektrolitów i nieelektrolitów
Chemical Quantities and Aqueous Reactions
62.0K Wyświetleń

4.7 : Rozpuszczalność związków jonowych
Chemical Quantities and Aqueous Reactions
62.1K Wyświetleń

4.9 : Reakcje wytrącania
Chemical Quantities and Aqueous Reactions
49.9K Wyświetleń

4.10 : Reakcje utleniania-redukcji
Chemical Quantities and Aqueous Reactions
63.9K Wyświetleń

4.11 : Stopnie utlenienia
Chemical Quantities and Aqueous Reactions
36.5K Wyświetleń

4.12 : Kwasy, zasady i reakcje neutralizacji
Chemical Quantities and Aqueous Reactions
54.2K Wyświetleń

4.13 : Reakcje syntezy i rozkładu
Chemical Quantities and Aqueous Reactions
31.9K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone