6.5 : Quantifying Work
As a system undergoes a change, its internal energy can change, and energy can be transferred from the system to the surroundings, or from the surroundings to the system.
Energy transfer occurs through heat and work. The relationship between internal energy, heat, and work is represented by the equation:
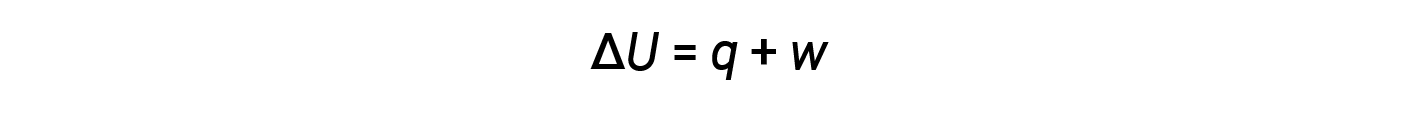
While heat is a function of an observed temperature change, work is a function of an observed volume change called the pressure-volume work. Work (w) can be defined as a force (F) acting through a distance (D).
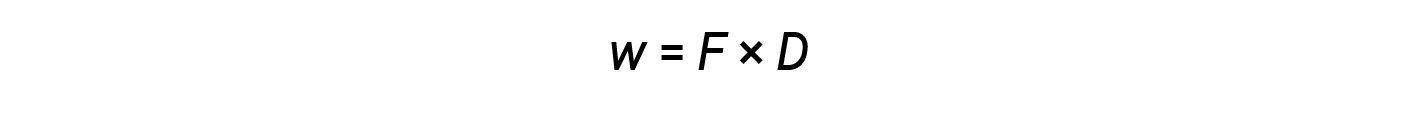
Pressure-volume work (or expansion work) occurs when a system pushes back the surroundings against a restraining pressure, or when the surroundings compress the system. An example of this occurs during the operation of an internal combustion engine. The combustion reaction of gasoline and oxygen is exothermic. Some of this energy is given off as heat, and some is performed as work by expanding the gases in the cylinder, thereby pushing the piston outward. The substances involved in the reaction are the system, and the engine and the rest of the universe are the surroundings. The system loses energy by both heating and doing work on the surroundings, and its internal energy decreases.
When the volume of a cylinder increases (i.e., the gas expands), it pushes against an external force, which is the pressure defined as force per unit area.
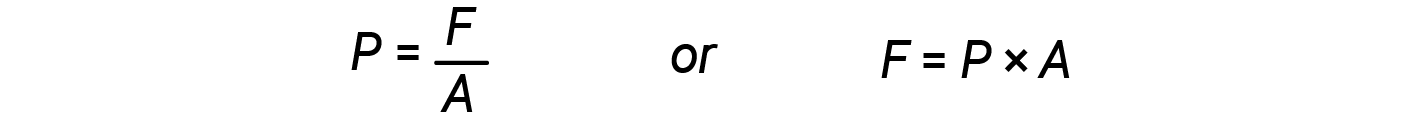
From equations 2 and 3:
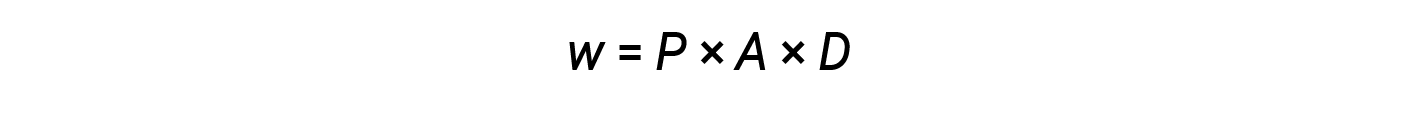
The product of area and distance (A × D) is equal to the change in the volume (ΔV) of the gas in the cylinder.
Therefore,

Since the volume increases during expansion, Vfinal > Vinitial, and ΔV is positive. However, for a positive expansion (i.e., when the system does work on the surroundings), w should be negative, and therefore, a negative sign is added to the equation.
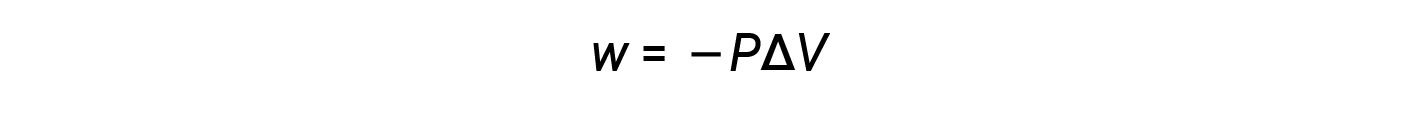
According to this equation, pressure-volume work is the negative of the external pressure (or opposing pressure) multiplied by the change in volume.
The unit of work based on this equation is L·atm. Some other useful conversions factors are:
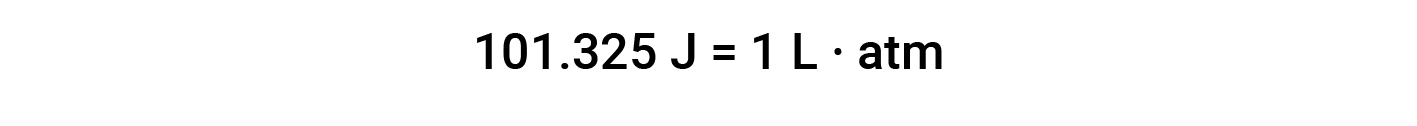
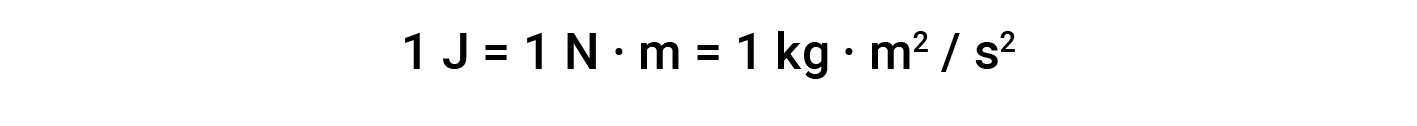
This text is adapted from Openstax, Chemistry 2e, Section 5.3: Enthalpy.
Z rozdziału 6:

Now Playing
6.5 : Quantifying Work
Thermochemistry
19.1K Wyświetleń

6.1 : Podstawy energetyki
Thermochemistry
37.1K Wyświetleń

6.2 : Pierwsza zasada termodynamiki
Thermochemistry
31.6K Wyświetleń

6.3 : Energia wewnętrzna
Thermochemistry
28.8K Wyświetleń

6.4 : Kwantyfikacja ciepła
Thermochemistry
53.9K Wyświetleń

6.6 : Entalpia
Thermochemistry
34.8K Wyświetleń

6.7 : Równania termochemiczne
Thermochemistry
28.3K Wyświetleń

6.8 : Kalorymetria stałociśnieniowa
Thermochemistry
84.7K Wyświetleń

6.9 : Kalorymetria stałoobjętościowa
Thermochemistry
26.9K Wyświetleń

6.10 : Prawo Hessa
Thermochemistry
44.5K Wyświetleń

6.11 : Standardowa entalpia formacji
Thermochemistry
41.1K Wyświetleń

6.12 : Entalpie reakcji
Thermochemistry
31.5K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone