Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Biliary System Resin Casting: A Technique for Analyzing 3D Architecture of Biliary System in Murine Model Using Radiopaque Resin Injection
W tym Artykule
Overview
This video describes the procedure for biliary system resin casting, for understanding 3D biliary architecture. This technique provides insights into the internal diameter and network connectivity of the biliary system, which could be used for liver regeneration analysis.
Protokół
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Double resin injections
- Preparation
- Prepare resin solution. For double system resin injections, prepare both yellow and green resins as described in the steps 1.1.2-1.1.4.
- Prepare 1 mL aliquots of diluted resin per mouse.
- Yellow Resin: Dilute yellow silicone rubber (high radiopacity) with the clear diluent in a 3:1 dilution to prepare yellow resin for injection.
- Green Resin: Mix blue silicone rubber (undetectable radiopacity) with the clear diluent in a 1:1 dilution to prepare blue resin. To generate green resin, mix the diluted blue resin with the diluted yellow resin at a ratio of 1:1. Vortex the green resin thoroughly until the color is homogeneous.
NOTE: Blue resin has very low radiopacity that is not detectable with microCT, which therefore necessitates dilution with yellow resin to create two resins with different radiopacities.
CAUTION: Resin may contain lead chromate, which is a carcinogen. It produces poisonous gases in a fire. Handle with care and dispose of the resin as hazardous waste. - Prepare two injection sets (injection set #1 and injection set #2) with tubing as described in the steps 1.1.6-1.1.11.
NOTE: For adult mice (>P30 (postnatal day 30) = P30 - 2 years), use injection set #1 (PE10 tubing with 0.6 mm outer diameter) for common bile duct injection and injection set #2 (PE50 tubing with 0.96 mm outer diameter) for portal vein injection. For young postnatal mice (up to P30), prepare two #1 injection sets, one for bile duct and one for portal vein injection (no injection set #2 as the portal vein is too narrow to accommodate PE50 tubing). - To prepare injection set #1, cut 30 cm of PE10 tubing and stretch one end of the tubing by hand by pulling it until it becomes as thin as possible (Figure 1A,B, approximately 0.15 mm diameter, non-stretched PE10 tubing is 0.6 mm diameter).
- Cut the tip of the stretched PE10 tubing diagonally to create a beveled tip (Figure 1B).
- Connect the non-stretched end of the PE10 tubing to a 30 G needle (Figure 1A, Injection set #1).
- To prepare injection set #2, cut 30 cm of PE50 tubing and stretch one end of the tubing by hand by pulling it until it becomes thin enough to fit into the portal vein (Figure 1A,B, approximately 0.7 mm, non-stretched PE50 tubing is 0.96 mm diameter).
- Cut the tip of the stretched PE50 tubing diagonally to create a beveled tip (Figure 1B).
- Connect the non-stretched end of the PE50 tubing to a 23 G needle (Figure 1A, injection set #2).
NOTE: Each injection set can only be used for one injection since the resin will harden in the syringe and tubing. Adjust the size of the stretched and beveled tip based on the size of the common bile duct and portal vein of the mouse, depending on its age, genotype, and phenotype. When unsure about the size and fit of the tubing, insert the tubing in the appropriate duct or vessel after the incision and before the tubing is filled with resin. If the tubing is too wide to fit in the duct/vessel, stretch it further. - Anesthetize the animal by isoflurane inhalation (first 4% in the induction chamber and ~2% using the nose cone).
- Place the mouse on a ventilated bench on a dissection pad while the mouse is breathing isoflurane through the nose cone. Verify that the animal is unconscious by an institute/IRB-recommended method, e.g., by pinching one of the paws.
CAUTION: Isoflurane may cause drowsiness or dizziness upon inhalation and may cause damage to the cardiovascular system and central nervous system through prolonged or repeated exposure. Do not inhale it. Handle the substance on a ventilated bench and in well-ventilated areas.
NOTE: It is possible to use another anesthetizing method, as long as it is compatible with cardiac perfusion. - Once the animal is unconscious, spray the ventral side with 70% ethanol to prevent interference from fur.
- Using skin scissors, cut the skin, fascia, and muscle layer starting at the midline of the abdominal cavity and cut through to the thoracic cavity to expose the internal organs.
- Grab the xiphoid process with forceps to lift the sternum and cut the diaphragm and rib cage on both sides to expose the heart and lungs.
- Remove the rib cage by cutting through the ribs on the left and right sides of the ribcage with scissors. Take extra care not to damage the liver as this will result in leakage of the resin.
- Using straight forceps, pull the heart towards the liver and cut away the right atrium.
- Insert a butterfly needle (23 G), connected to a peristaltic perfusion pump, into the left ventricle. Perfuse the mouse transcardially with Hanks balanced salt solution (HBSS) and heparin (1 U/g of mouse body weight). Perfuse for 3 min with a perfusion rate of 5 mL/min.
NOTE: If the mouse is being perfused correctly, the internal organs will become pale, especially the liver. If, instead, the lungs are turning white, the needle is inserted into the right ventricle and should be repositioned. After perfusion, the mouse is exsanguinated and can be removed from the nose cone and isoflurane. - Turn off the isoflurane pump.
2. Resin injection - Biliary system resin casting
- Move the mouse to the dissection microscope, abdomen up, tail towards the experimenter, and head away from the experimenter. The anatomical landmarks of interest are depicted in Figure 2A.
- Locate the inferior vena cava (Figure 2A) by moving the intestine to the side. Use spring scissors to make a small transversal incision in the inferior vena cava to allow the release of hepatic vascular pressure (Figure 2Bi).
- Expose the common bile duct and portal vein as described in steps 1.2.4- 1.2.6.
- Move the intestine and pancreas to the right side (of the experimenter) using a phosphate-buffered saline (PBS)-wetted cotton swab.
- Flip the ventral side of the liver towards the heart using the PBS-wetted cotton swab to expose the visceral surface and the hilar region.
- Locate the common bile duct that runs from the hilar region across the pancreas and into the intestine at the sphincter of Oddi (Figure 2Bii, common bile duct outlined with the yellow dotted line, black arrowheads point to Sphincter of Oddi).
NOTE: Make sure the liver is moist throughout the procedure by sprinkling it with PBS. - Clear surrounding tissue (area ~5 mm) from the common bile duct using straight forceps. Place silk suture thread (size 4-0, 0.17 mm, 3-5 cm long) under the common bile duct (Figure 2Bii) and tie a loose overhand knot around the common bile duct (Figure 2Biii).
NOTE: Choose an area for the knot halfway between the hilar region and the sphincter of Oddi and at some distance from the portal vein so that after the suture is tightened around the common bile duct, it will not interfere with the portal vein injection. - Hold the spring scissors flat against the common bile duct to make an oblique incision to the common bile duct at the spot where the common bile duct enters the pancreas and intestine next to the sphincter of Oddi. (Figure 2Biv), yellow dotted line outlines the sphincter of Oddi region, emphasized by black arrowheads).
NOTE: This is a crucial step. Make an oblique and not a transversal cut, and make an incision; do not sever the bile duct. Cutting through the entire bile duct makes insertion of the tubing very challenging. - Just before use, mix 1 mL of the yellow resin with 50 µL of the curing agent (by filling and emptying the 1 mL syringe) and fill a 1 mL Luer syringe with the resin-curing agent mixture.
- Connect the filled syringe with the tubing (set #1). Press the plunger to fill the tubing completely. Ensure that the resin/curing agent mix drips from the tip of the tubing.
NOTE: Avoid and remove bubbles in the syringe and tubing for best results. - Using forceps, straighten the area around the common bile duct incision and insert the tubing into the opening in the common bile duct (Figure 2Bv), with the longest edge of the beveled tip downwards towards the dorsal side of the bile duct. This orientation ensures that resin can exit the tubing opening, which is facing upwards, into the duct (Figure 2Bv).
- Tighten the silk thread knot to secure the tubing inside the common bile duct (Figure 2Bvi).
- Inject the resin into the common bile duct. Observe the gall bladder and the individual liver lobes.
- Massage the liver with a PBS-wetted cotton swab to help spread the resin equally. Resin-filled bile ducts' terminal branches (in wild-type mice) are faintly visible at the liver surface.
NOTE: Expected time to fill the liver is 30-100 s. - Stop injecting the resin when dots of resin appear at the surface of the liver (Figure 2Ci, blue arrowheads) or when resistance is met.
NOTE: Work as fast as possible since the resin begins to harden after adding the resin curing agent. The working time from adding the curing agent is approximately 15 min. - Remove the tubing by pulling it out of the common bile duct and quickly tighten the silk knot using forceps to prevent the resin from leaking out. Cut away the loose ends of the silk suture, so they do not interfere with the portal vein injection.
- Dispose of the tubing containing resin and the remaining resin into the hazardous waste and the needle into the sharps waste.
Wyniki
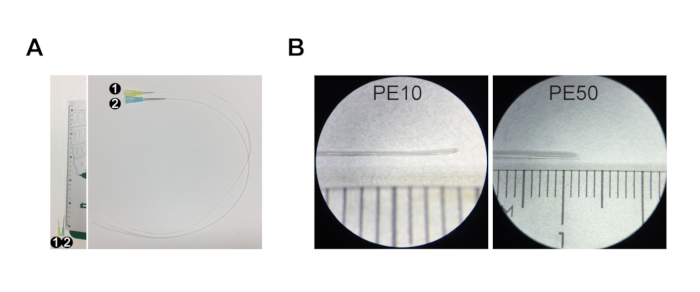
Figure 1: Injection set for resin casting. (A) Injection set #1 comprises a 30 G needle and PE10 tubing that is ~30 cm long. Injection set #2 is composed of a 23 G needle and PE50 tubing ~30 cm long. (B) The tip of the tubing is stretched and cut at an angle to create a beveled tip. The ruler in A and B is a centimeter ruler, with major increments of 1 cm, i...
Ujawnienia
Materiały
| Name | Company | Catalog Number | Comments |
| 1.5 mL SafeSeal micro tubes | Sarstedt | 72.706 | |
| 23 G butterfly needle with tubing | BD bioscience | 367283 | |
| 23 G needle | BD bioscience | 305892 | |
| 30 G needle | BD bioscience | 305106 | |
| Agarose | Top-Bio | P045 | |
| Benzyl alcohol | Sigma Aldrich | 108006 | |
| Benzyl benzoate | Sigma Aldrich | B6630 | |
| Corning 50 mL tubes | Sigma Aldrich | CLS430829-500EA | polypropylene |
| Cotton swabs | Medicarier | 60406 | |
| Dissection Microscope | Leica Camera AG | Leica M60 | |
| Dulbecco's phosphate-buffered saline | ThermoFisher Scientific | 14190144 | |
| Ethanol 70% | VWR | 83801.41 | |
| Falcon tube 15 mL | Verkon | 331.850.084.006 | |
| Forceps curved | Fine Science Tools | 11051-10 | Fine Graefe 10 cm curved |
| Forceps straight | Fine Science Tools | 11050-10 | Fine Graefe 10 cm straight |
| Formaldehyde solution | Sigma Aldrich | F8775 | |
| Hanks' Balanced Salt Solution | ThermoFisher Scientific | 14025092 | |
| Heparin | Leo Pharma | B01AB01 | 5000 IE/mL |
| Isolfurane | Baxter | FDG9623 | |
| Methanol | ThermoFisher Scientific | 11413413 | |
| MICROFIL | Flowtech | MV-122 | synthetic resin yellow |
| MICROFIL | Flowtech | MV-120 | synthetic resin blue |
| MICROFIL | Flowtech | MV-diluent | clear resin diluent |
| Pasteur pipette | Verkon | 130.690.424.503 | |
| Peristaltic pump | AgnThos | 010.6131.M20 | |
| Rocker | VWR | 444-0142 | |
| Silk suture | AgnThos | 14757 | Black silk, 4-0, sterile, 100 m |
| Skin scissor | Fine Science Tools | 14058-09 | Iris straight tip 9 cm |
| Spring scissor | Fine Science Tools | 15000-03 | Vannas micro, straight tip 2 mm |
| Syringe 1 mL Luer | BD bioscience | 303172 | |
| Tubing PE10 | BD bioscience | 427401 | |
| Tubing PE50 | BD bioscience | 427411 |
This article has been published
Video Coming Soon
Source: Hankeova, S. et al. DUCT: Double Resin Casting followed by Micro-Computed Tomography for 3D Liver Analysis. J. Vis. Exp. (2021)
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone