Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Generating Postganglionic Sympathetic Neurons from Human Pluripotent Stem Cells
W tym Artykule
Overview
This video demonstrates an in vitro method to generate postganglionic sympathetic neurons from human pluripotent stem cells (hPSCs). hPSCs are differentiated into neural crest cells (NCCs) on a basement membrane matrix support using a differentiation medium. The NCCs are then dissociated and formed into spheroids using another differentiation medium. Finally, the spheroids are transformed into postganglionic sympathetic neurons using a sympathetic neuron medium.
Protokół
1. Set-up for dish coating, media preparation, and hPSC maintenance
- Dish coating
- Vitronectin (VTN) coating
- Place vials of VTN in a 37 °C water bath until fully thawed, then mix thoroughly.
- For a 100 mm Petri dish, mix 7 mL of 1x phosphate buffered saline (PBS) with 0.5 mg/mL VTN, add VTN solution to the dish, and incubate at room temperature (RT) for 1 h.
- Basement membrane matrix coating
- Thaw vials of basement membrane matrix (see Table of Materials) on ice at 4 °C overnight.
- For one well of a 6 well plate, mix 2 mL of Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) with 20 µL of 100x basement membrane matrix, add basement membrane matrix solution to the dish, wrap the dish with paraffin film, and store in a clean container at 4 °C overnight. Work as quickly as possible. Coated dishes can be stored in 4 °C for up to 2 weeks.
- Polyornithine (PO)/laminin (LM)/fibronectin (FN) coating
- On the first day, for one well of a 24 well plate, mix 15 µg/mL of PO with 1 mL of 1x PBS, incubate at 37 °C, 5% CO2 overnight. Thaw both LM and FN at -20 °C overnight and store at 4 °C until fully thawed.
- On the second day, aspirate PO solution, wash the wells 2x with 1x PBS, add 1 mL of 1x PBS containing 2 µg/mL of LM and 2 µg/mL of FN and incubate at 37 °C in 5% CO2 overnight. At this point the dish with the LM/FN solution can be kept in the incubator for months as long as it does not dry out. Add more 1x PBS to prevent the dish from drying out.
- Vitronectin (VTN) coating
- Media preparation
- Prepare the Essential 8 medium (E8) by thawing one bottle of E8 supplement at 4 °C overnight. Mix the supplement with 500 mL of E8 medium and antibiotics if needed.
NOTE: Working E8 solution should be used up within 2 weeks. - Prepare the hPSC freezing medium by mixing 90 mL of complete E8 medium with 10 mL of dimethyl sulfoxide (DMSO) for a total volume of 100 mL. Filter sterilize.
- Prepare the day 0 to day 1 differentiation medium by mixing 100 mL of essential 6 (E6) medium with 10 µM SB431542, 1 ng/mL bone morphogenetic protein 4 (BMP4), 300 nM CHIR99021, and 10 µM Y27632 for a total volume of 100 mL.
- Prepare the day 2 to day 10 differentiation medium by mixing 100 mL of E6 medium with 10 µM SB and 0.75 µM CHIR99021 for a total volume of 100 mL.
- Prepare the day 10 to day 14 spheroid medium by mixing neurobasal medium with 2 mL of B27 (50x), 1 mL of N2 (100x), 2 mM L-Glutamate, 3 µM CHIR99021, and 10 ng/mL fibroblast growth factor-2 (FGF2) for a total volume of 100 mL.
- Prepare the day 14 to day 28 medium for spheroid long term maintenance by adding 0.5 µM of fresh retinoic acid (RA) to the day 10 to day 14 spheroid medium for every feeding.
NOTE: Always keep RA at -80 °C. - Prepare the SymN maturation medium by mixing neurobasal medium with 2 mL of B27 (50x), 1 mL of N2 (100x), 2 mM L-glutamate, 25 ng/mL glial cell line-derived neurotrophic factor (GDNF), 25 ng/mL brain-derived neurotrophic factor (BDNF), 25 ng/mL nerve growth factor (NGF), 200 µM ascorbic acid, and 0.2 mM dibutyryl cyclic adenosine monophosphate (dbcAMP) for a total volume of 100 mL. The solution should be used within 2 weeks. Before each feeding, add 0.125 µM fresh RA. This solution is used from day 14 (option 1) or day 28 (option 2).
- Prepare the fluorescence-activated cell sorting (FACS) buffer by mixing DMEM with 2% FBS, 2 mM L-glutamate and antibiotics if needed for a total volume of 100 mL.
- Prepare the Essential 8 medium (E8) by thawing one bottle of E8 supplement at 4 °C overnight. Mix the supplement with 500 mL of E8 medium and antibiotics if needed.
- hPSC maintenance
- Thawing and keeping hPSCs
- Prepare one VTN coated 100 mm dish.
- To thaw a vial of hPSCs directly from liquid nitrogen, put the vial into a 37 °C water bath, carefully swinging the tube in the water until it thaws. Transfer the thawed hPSCs to a 15 mL tube containing 10 mL of 1x PBS, and centrifuge at 200 x g for 4 min.
- Discard the supernatant and add 1 mL of E8 medium to the tube. Pipette a few times to fully resuspend the pellet and then add another 9 mL of E8 medium to reach 10 mL total.
- Aspirate the VTN solution from the 100 mm dish.
- Transfer the hPSCs to a 100 mm dish, shake gently (up-down and left-right, not in circles) to make sure cells are distributed evenly in the dish
- Incubate at 37 °C, in 5% CO2.
- On the following day, aspirate all medium and feed with 10 mL of E8.
- Feed this way every day for the next 3–4 days and then prepare to split.
- Splitting hPSCs
NOTE: hPSCs at the point of splitting should be 80%–90% confluent. Big colonies with smooth and bright edges should be observed. However, contact between each colony should be avoided (Figure 1B, day 0 and Figure 4B).- Prepare VTN-coated 100 mm dishes as needed.
- Aspirate the E8 and wash the dish that needs to be split 1x with 1x PBS.
- Aspirate the 1x PBS and add 4 mL of 0.25 M EDTA. Incubate for 2 min at 37 °C, 5% CO2.
NOTE: The hPSCs should be split/replated as small colonies. Do not treat with ethylenediaminetetraacetic acid (EDTA) longer than 2 min to prevent separation into single cells. The cells should be still attached to the dish surface after the 2 min treatment. - Aspirate the EDTA, detach the colonies by strongly pipetting 10 mL of E8 medium onto the dish surface, and collect all the medium and cells in a 15 mL tube.
- With hPSCs at 80%–90% confluency, split colonies by 1:15-1:20. For example, to split hPSCs by 1:20 into one 100 mm dish, take 500 µL of E8/hPSCs solution and mix with 9.5 mL of fresh E8 medium.
- Plate hPSCs in VTN-coated 100 mm dishes.
NOTE: It is advised to establish the ideal split ratio for each researcher and cell line independently.
- Freezing hPSCs
- For one 100 mm dish of hPSCs that is ready to be split, prepare three cryovials and 3.5 mL of freezing medium.
NOTE: Media and vials should be kept in 4 °C or on ice until usage. - Aspirate E8 and wash the dish 2x with 1x PBS.
- Aspirate 1x PBS and add 4 mL of 0.25 M EDTA, incubate for 2 min at 37 °C, in 5% CO2.
NOTE: hPSCs should be frozen as small colonies at the time that they would be split. Do not treat the cells with EDTA longer than 2 min to prevent separation into single cells. Cells should be still attached on the dish's surface after the 2 min treatment. - Aspirate the EDTA, detach the colonies by strongly pipetting 10 mL of 1x PBS on the dish's surface, and collect all medium and cells in a 50 mL tube.
- Add 20 mL of 1x PBS and centrifuge at 200 x g for 4 min to wash out the remaining EDTA.
- Discard the supernatant and resuspend the pellet in 3 mL of freezing medium.
- Distribute hPSCs evenly into the three cryovials, 1 mL each.
- Store at -80 °C overnight in a controlled freezing box or a styrofoam sandwich to ensure a slow temperature drop, and then transfer to a liquid nitrogen tank for long term storage.
- For one 100 mm dish of hPSCs that is ready to be split, prepare three cryovials and 3.5 mL of freezing medium.
- Thawing and keeping hPSCs
2. Seeding hPSCs to start the differentiation (day 0)
NOTE: hPSCs should be ready for differentiation after being stabilized (i.e., being split 2–3x after thawing). Be sure that the colonies are healthy, with smooth, shiny edges, and minimal differentiation (Figure 2B).
- Prepare basement membrane matrix-coated dishes (24 well or 6 well dishes) one day before day 0 as needed. Bring the dishes to RT at the start of differentiation.
- Make the day 0–1 differentiation medium as needed.
- Aspirate the E8 from the confluent, ready to split hPCSs, and wash the dish 2x with 1x PBS.
- Add 7 mL of 0.25 M EDTA per 100 mm dish, incubate at 37 °C, 5% CO2, for 15 min.
NOTE: At this point, EDTA treatment is prolonged to disperse into single cells. - Pipet off all the hPSCs (they should be floating) and transfer to a 50 mL tube. Add the same amount or more of 1x PBS as EDTA solution to dilute out the EDTA.
- Centrifuge at 200 x g for 4 min.
- Discard the supernatant, add 1 mL of day 0–1 differentiation medium and pipet to homogenize the cells. Follow by adding more medium and then mix to dilute the cell solution to a concentration ideal to count the cells.
NOTE: Do not overdilute the cell solution. Cells from one full 100 mm dish should be diluted in 5 mL of medium to start. - Count the cell number using an automated cell counter or hemocytometer.
- Dilute the cell solution as needed to reach 125,000 cells/cm2 in a low final volume (e.g., 2 mL per well for a 6 well dish or 500 µL per well for a 24 well dish).
NOTE: A low volume helps the cells attach faster. - Aspirate all of the basement membrane matrix solution from the coated dishes and plate the cell solution into the wells.
- Incubate at 37 °C, in 5% CO2.
3. Neural crest cell induction (day 1 to day 10, Figure 1A)
- On day 1 feed the cells with day 0–1 differentiation medium (3 mL per well for 6 well dishes and 1 mL per well for 24 well dishes).
- On day 2 feed the cells with day 2–day 10 differentiation medium (3 mL per well for 6 well dishes and 1 mL per well for 24 well dishes).
- From now on, the cells should be fed every other day until day 10 (i.e., the next feeding day will be day 4).
NOTE: From day 6 on, NC ridges should be detected (Figure 1B). To check if differentiation is taking place, it is advised to carry a parallel differentiation culture in smaller wells (i.e., 24 wells), that can be stained for SOX10/AP2a and used for marker gene expression along the time of differentiation (Figure 1B,C). - If sorting cells, proceed to section 4. Otherwise, proceed to section 5.
4. Fluorescence activated cell sorting (FACS) for neural crest marker CD49D and aggregating NC cells in spheroids
NOTE: For FACS sorting, the samples should be kept on ice and not be exposed to light after staining until sorting.
- Prepare FACS buffer if the cells are sorted.
- Prepare day 10–14 spheroid medium.
- On day 10, remove the medium, and wash 1x with 1x PBS.
- Add dissociation solution (see Table of Materials) at 2 mL per well for a 6 well dish or 1 mL per well for a 24 well dish, and incubate at 37 °C, 5% CO2 for 20 min.
- Pipet off all the hPSCs and transfer to a 50 mL tube.
- Fill up the rest of the tube with FACS buffer and centrifuge at 200 x g for 4 min.
NOTE: Each 50 mL tube can accommodate up to 20 mL or less of cell solution. The volume of the FACS buffer should be high enough to neutralize the dissociation solution. - Discard the supernatant, resuspend the cells with the appropriate amount of FACS buffer (~2 mL per well of a 6 well plate), and count to determine the cell number.
- Prepare the following samples.
- Sample 1 (unstained control): 1 x 106 cells in 400 µL of FACS buffer. Filter the cells through a 20 µm strainer cap and keep the tube on ice.
- Sample 2 (DAPI only control): 1 x 106 cells in 400 µL of FACS buffer containing 0.5 ug/mL 4′,6-diamidino-2-phenylindole (DAPI). Filter the cells through a FACS tube with a strainer cap and keep the tube on ice.
- Sample 3 (CD49D-labeled): Suspend the rest of the cells with FACS buffer containing phycoerythrin/cyanine7 (PE/Cy7)-conjugated CD49D antibody (5 µL for 1 x 106 cells per 100 µL of FACS buffer) in a 15 mL tube and incubate on ice for 20 min.
- Fill up the tubes with FACS buffer and centrifuge at 200 x g for 4 min.
- Discard the supernatant and resuspend every 5–10 x 106 cells in 1 mL of FACS buffer containing 0.5 ug/mL DAPI according to the manufacturer's instructions.
- Filter the cells through the FACS tube with the strainer cap and keep the tube on ice.
- Prepare collection FACS tubes containing 2 mL of FACS buffer.
- Sort through the FACS machine with lasers that can detect DAPI and PE-Cy7 to isolate the CD49D+ population.
- After sorting, count the sorted cells.
- Centrifuge all sorted cells and resuspend in day 10–14 spheroid medium to a final concentration of 0.5 x 106 cells per 500 µL of medium.
- Plate 0.5 x 106 cells per well into ultra-low attachment 24 well plates.
- Incubate the cells at 37 °C, in 5% CO2.
5. Aggregating NC cells in spheroids
- If not using FACS to isolate the NC cells and instead aggregating them into spheroids directly, first prepare cells as described in steps 4.2–4.5.
- Fill up the rest of the tube with 1x PBS, and centrifuge at 200 x g for 4 min.
- Discard the supernatant, resuspend the cells with an appropriate amount of day 10–14 spheroid medium (e.g., ~2 mL of medium per well for a 6 well plate), and count to determine the cell number.
- Dilute the cells in day 10–14 spheroid medium to 0.5 x 106 cells per 500 µL of medium.
- Plate 500 µL of the cell suspension per well in ultra-low attachment 24 well plates.
- Incubate the cells at 37 °C, in 5% CO2.
6. NC spheroid maintenance and sympathetic progenitor induction (day 10 to day 14, Figure 3A)
- Option 1: Minimal spheroid culture
- On day 11, add 500 µL of day 10–14 spheroid medium to the NC spheroids without aspirating existing medium from day 10. Incubate at 37 °C, in 5% CO2.
- On day 12, tilt the plate to accumulate the NC spheroids on one side of the wells. Carefully aspirate and discard as much medium as possible, and feed with 1 mL of day 10–14 spheroid medium.
- Keep feeding the cells every day until day 14.
- Optional: If the spheroids aggregate and generate a large clump, use a pipette to break the spheroid clumps up. This also ensures that individual spheroids do not get too large.
NOTE: The ideal spheroid size range should be around 100–500 μm. Within that range, the size of individual spheroids is not critical. However, the morphology, such as a smooth and clear edges (Figure 2 and Figure 4) is important for further success. At day 14, each 24 well plate ideally contains about 50–60 spheroids of different sizes within the abovementioned size range.
- Option 2: Expanded spheroid culture
- On day 15, to keep NC spheroids, feed with 1.5 mL of day 10–14 spheroid medium containing 0.5 µM RA. Incubate at 37 °C, 5% CO2.
NOTE: RA should be added fresh for every feeding and always be stored at -80 °C. - From now on, feed every other day up to day 28 and then continue with plating of the spheroids (section 7.1).
NOTE: The growing spheroids are split approximately 1x per week by pipetting them with a 1 mL pipette to break them up. They are split at an approximate ratio of 1:2–1:4. Within the 2 week expansion period, the cells should roughly quadruple in number.
- On day 15, to keep NC spheroids, feed with 1.5 mL of day 10–14 spheroid medium containing 0.5 µM RA. Incubate at 37 °C, 5% CO2.
7. Sympathetic neuron (SymN) differentiation and maturation (Option 1: after day 14; Option 2: after day 28)
- Plating spheroids in regular dishes
- Prepare PO/LM/FM-coated 24 well plates.
- Prepare symN medium containing 0.125 µM RA (add fresh every feed) and 10 µM Y27632 (day 14 only).
- On day 14, tilt the plate to accumulate NC spheroids on one side of the wells. Carefully aspirate and discard as much medium as possible, and feed with 1 mL of symN medium.
- Remove LM/FN from the coated plates.
- Split and plate each well from the 24 well plate into 4 separate wells of the new, coated 24 well plate. Each original well will have 1 mL, containing ~50–60 spheroids. This yields 250 µL, containing approximately 10–15 spheroids for each well on the new plate.
- Add 250 µL of additional medium per well.
NOTE: This is a split of 1:4; make sure that the spheroids are distributed properly within the solution so that the split is relatively even. The number of spheroids is not counted because the final number does not affect the success of generating symNs. - Incubate at 37 °C, 5% CO2.
- On day 15 (or day 29 for option 2), feed by replacing all medium with 1 mL of symN medium containing 0.125 µM RA. From now on, the neurons should be fed every 2 days until day 20 (or day 35 for option 2).
- After day 20 (or day 35 for option 2), the neurons should be fed by carefully replacing only half of the existing medium (500 µL). From now on, feed every week unless the medium quickly turns yellow.
- Keep feeding weekly until the desired time point.
NOTE: symNs tend to aggregate in ganglia-like structures and are prone to detach from the culture dishes. To prevent this, half-feedings and minimal handling is recommended.
Access restricted. Please log in or start a trial to view this content.
Wyniki
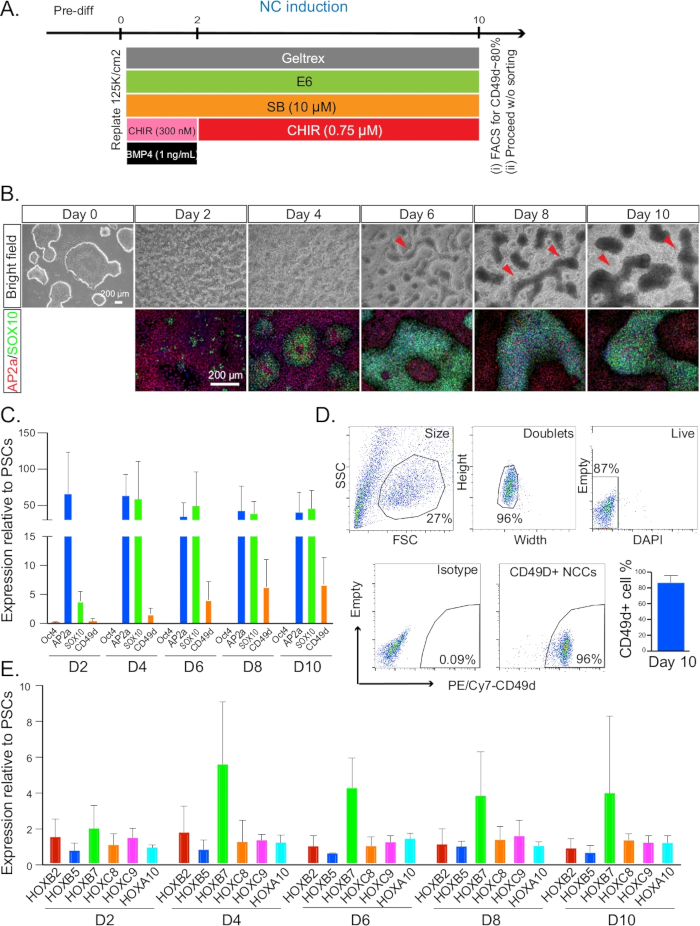
Figure 1: NC induction. (A) Timeline and treatments for NC induction from day 0 to day 10. (B) The morphology and formation of NC ridges were monitored every 2 days by bright field microscopy. Cells at each time point after day 2 were co-stained for AP2A (red) and SOX10 (green). All immunofluorescence pictures were counterstained with DAPI. Red arrows indicate the structures of ridges. (C) qRT-PCR analysis for the expression profile of NC markers from day 2–10. (D) Representative plot of FACS sorting on day 10 for CD49D+ NC populations (left). A typical gating strategy and isotype control is indicated in the first four plots. Quantification of CD49D+ cell percentage on day 10 was also performed. (E) qRT-PCR analysis for the expression profile of HOX genes from day 2–10. NC = neural crest. Error bars stem from data of n ≥ 3 biological repeats, defined as independent differentiation experiments done on separate days from PSC cultures that were at least one split apart.

Figure 2: Neural crest maintenance and expansion. (A) Timeline and treatments for NC expansion during day 10 to day 14 culture for option 1 or day 10 to day 28 culture for option 2. (B) The NC spheroid formation was monitored by bright field microscopy from day 11 (1 day after spheroid formation) to day 14 (i.e., the day of plating). Unsorted, CD49D+, and CD49D- populations were compared. The plated cells on day 15 are also shown. Cells failed to form spheroids properly in the CD49D- group and died after plating. Day 14 cells were examined by qRT-PCR analysis (right) for the expression profile of symN progenitors. Unsorted and CD49D+ populations were compared (n ≥ 3). (C) NC spheroids could be maintained for up to 2 weeks. Spheroids were monitored by bright field microscopy from day 15 to day 28 (the day of landing). The plated cells on day 29 are also shown. Day 28 spheroids were examined by qRT-PCR analysis for the expression profile of symN progenitors (right). Unsorted and CD49D+ populations were pooled (n ≥ 3).
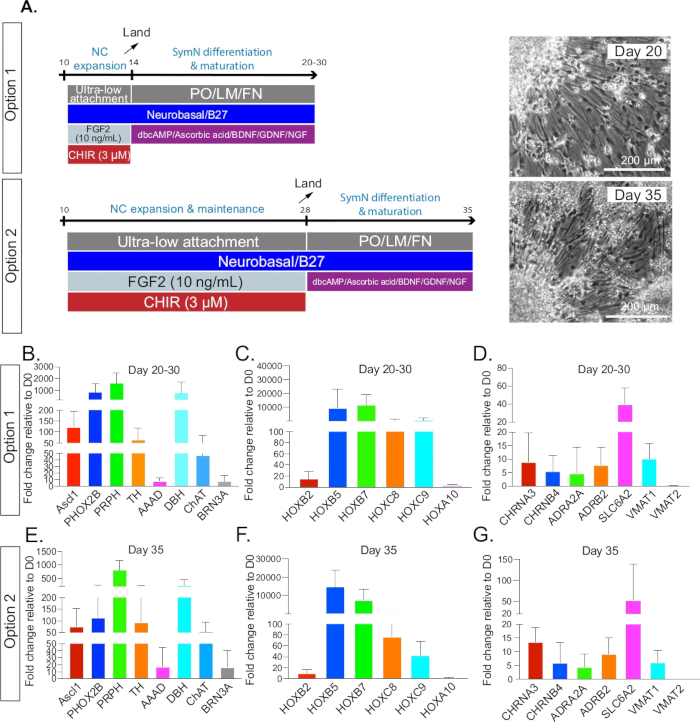
Figure 3: SymN differentiation and maturation. (A) Timeline and treatments after plating on day 14 for option 1 or day 28 for option 2. Bright field microscopy photos (right) show symNs on day 20 and day 35, respectively (1 week after plating for both options). (B–D) Option 1 qRT-PCR analysis for the expression profile of symN properties between days 20–30. Unsorted and CD49D+ populations were pooled. (E–G) Option 2 qRT-PCR analysis for the expression profile of symN properties after day 35. Unsorted and CD49D+ populations were pooled (n ≥ 3).
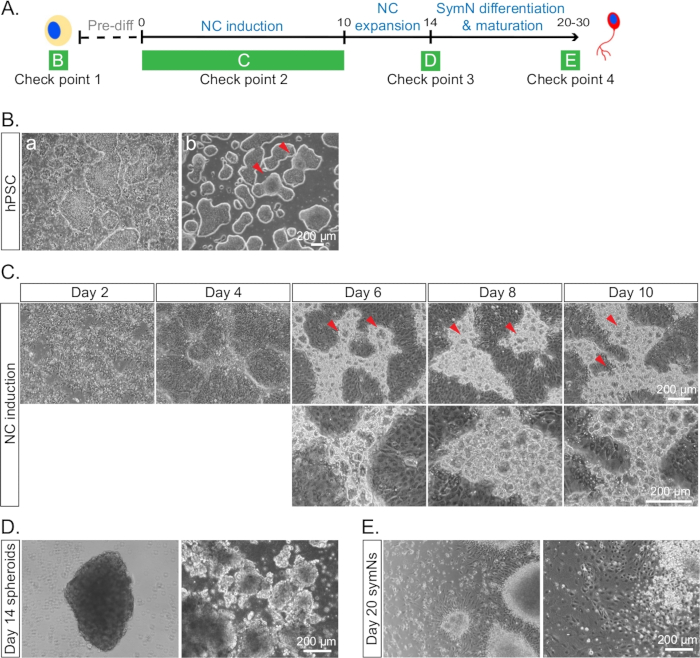
Figure 4: Example of cells under conditions that are inappropriate to proceed with the differentiation. (A) Timeline of the differentiation with each check point for cell morphologies indicated as in B–E. (B) hPSCs (a) with healthy colonies and a lot of differentiated cells, and (b) merged and differentiated borders between some colonies on day 0. Red arrows indicate the merged areas. (C) NCCs at day 10 with bubble-like blisters in the ridges. Red arrows indicate the blisters in the top row. The bottom row represents a higher magnification. We have not been able to identify the cell identity of the blisters. However, presence of more blisters seems to correlate with lower SOX10/CD49D expression. (D) Irregular looking spheroids lacking smooth edges and round shape on day 14. (E) Unhealthy and dying symNs on day 20.
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
Materiały
| Name | Company | Catalog Number | Comments |
| 100 mm cell culture dishes | Falcon | 353003 | |
| 15 mL conical tissue culture tubes | VWR/Corning | 89039-664 | |
| 24-well tissue culture plates | Falcon | 353047 | |
| 24-well ultra-low-attachment plates | Corning | 07 200 601 and 07 200 602 | |
| 5% CO2/20% O2 tissue culture incubator | Thermo Fisher/Life Technologies | Heracell VIOS 160i | |
| 50 ml conical tissue culture tubes | VWR/Corning | 89039-656 | |
| 6-well tissue culture plates | Costar | 3516 | |
| Accutase | Innovation Cell Technologies | AT104500 | Cell dissociation solution |
| Anti-AP2a antibody | Abcam | ab108311 | Host: Rabbit; 1:400 dilution |
| Anti-Ascl1 antibody | BD Pharmingen | 556604 | Host: Mouse IgG1; 1:200 dilution |
| Anti-CD49D antibody | BioLegend | 304313 | Host: Mouse IgG1; 5 μl/million cells in 100 μl volume |
| Anti-CD49D antibody (isotype) | BioLegend | 400125 | Host: Mouse IgG1; 5 μl/million cells in 100 μl volume |
| Anti-DAPI antibody | Sigma | D9542 | 1:1000 dilution |
| Anti-DBH antibody | Immunostar | 22806 | Host: Rabbit; 1:500 dilution |
| Anti-GFP antibody | Abcam | ab13970 | Host: Chicken; 1:1000 dilution |
| Anti-HOXC9 antibody | Santa Cruz Biotechnology | sc-365692 | Host: Mouse IgG1; 1:100 dilution |
| Anti-NET1 antibody | Mab | NET17-1 | Host: Mouse; 1:1000 dilution |
| Anti-PRPH antibody | Santa Cruz Biotechnology | SC-377093/H0112 | Host: Mouse IgG2a; 1:200 dilution |
| Anti-SOX10 antibody | Abcam | ab50839 | Host: Mouse; 1:100 dilution |
| Anti-TH antibody | Pel-Freez | P40101- 150 | Host: Rabbit; 1:500 dilution |
| Ascorbic acid | Sigma | A8960-5G | Stock concentration: 100 mM |
| B27 supplement | Thermo Fisher/Life Technologies | 12587-010 | Stock concentration: 50x |
| BDNF | R&D Systems | 248-BD | Stock concentration: 10 μg/mL |
| BMP4 | R&D Systems | 314-BP | Stock concentration: 6 mM |
| Cell counter | Thermo Fisher/Life Technologies | Countess II | |
| Cell counting chamber slides | Invitrogen | C10312 | |
| Centrifuge | Eppendorf | 57021&5424R | |
| CHIR99021 | R&D Systems | 4423 | Stock concentration: 6 mM |
| Cryo-vial | Thermo Fisher/Life Technologies | 375353 | |
| dbcAMP | Sigma | D0627 | Stock concentration: 100 mM |
| DMEM | Thermo Fisher/Life Technologies | 10829-018 | Stock concentration: 1x |
| DMEM/F12 | Thermo Fisher/Life Technologies | 11330-057 | Stock concentration: 1x |
| DMSO | Thermo Fisher/Life Technologies | BP231-100 | |
| E6 medium | gibco | A15165-01 | |
| E8 medium | gibco | A15169-01 | Stock concentration: 1x |
| E8 supplement | gibco | A15171-01 | Stock concentration: 50x |
| EDTA | Sigma | ED2SS | Stock concentration: 0.5 M |
| FACS machine | Beckman Coulter | CytoFLEX (for FACS) | |
| FACS machine | Beckman Coulter | MoFlo Astrios EQ (for sorting) | |
| FACS tubes (blue filter cap) | Falcon | 352235 | |
| FACS tubes (white cap) | Falcon | 352063 | |
| Fetal bovine serum (FBS) | Atlanta Biologicals | S11150 | |
| GDNF | PeproTech | 450 | Stock concentration: 10 μg/mL |
| Geltrex | Invitrogen | A1413202 | Basement membrane matrix; Stock concentration: 100x |
| hPSCs | Thomson et al., (1998) | WA09 | |
| hPSCs | Oh et al. (2016) | H9-PHOX2B::eGFP | |
| Human fibronectin (FN) | VWR/Corning | 47743-654 | Stock concentration: 1 mg/mL |
| L-glutamine | Thermo Fisher/Gibco | 25030-081 | Stock concentration: 200 mM |
| LN tank | Custom Biogenic Systems | V-1500AB | |
| Mouse laminin I (LM) | R&D Systems | 3400-010-01 | Stock concentration: 1 mg/mL |
| N2 supplement | Thermo Fisher/Life Technologies | 17502-048 | Stock concentration: 100x |
| Neurobasal medium | gibco | 21103-049 | Stock concentration: 1x |
| NGF | PeproTech | 450-01 | Stock concentration: 25 μg/mL |
| Phosphate-buffered saline (PBS) | Gibco | 14190-136 | Stock concentration: 1x |
| Poly-L-ornithine hydrobromide (PO) | Sigma | P3655 | Stock concentration: 15 mg/mL |
| Primocin (antibiotics) | InvivoGen | ANTPM1 | Stock concentration: 50 mg/mL |
| qPCR machine | Bio-Rad Laboratories | C1000 Touch | |
| qPCR plates | Bio-Rad Laboratories | HSP9601 | |
| recombinant FGF2 | R&D Systems | 233-FB/CF | Stock concentration: 10 μg/mL |
| Retinoic acid | Sigma | R2625 | Stock concentration: 1 mM |
| SB431542 | Tocris/R&D Systems | 1614 | Stock concentration: 10 mM |
| Trypan blue | Corning | MT-25-900-CI | |
| Vitronectin (VTN) | Thermo Fisher/Life Technologies | A14700 | Stock concentration: 0.5 mg/mL |
| Water bath | VWR/Corning | 706308 | |
| Y27632 | R&D Systems | 1254 | Stock concentration: 10 mM |
Odniesienia
Access restricted. Please log in or start a trial to view this content.
This article has been published
Video Coming Soon
Source: Wu, H. F., et al. Efficient Differentiation of Postganglionic Sympathetic Neurons using Human Pluripotent Stem Cells under Feeder-free and Chemically Defined Culture Conditions. J. Vis. Exp. (2020).
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone