Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Electrophysiological Recordings of Brainstem-Spinal Cord Preparations from a Newborn Rodent
W tym Artykule
Overview
This video demonstrates electrophysiological recordings of isolated brainstem-spinal cord preparations from newborn rodents. The preparation is placed in a recording chamber, and the inspiratory bursts from the brainstem are recorded under oxygenated and oxygen-deprived conditions using the fourth ventral nerve root in the spinal cord.
Protokół
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Setup and Preparation
- Solutions
- Prepare artificial cerebrospinal fluid (aCSF) stock solutions according to the following recipes. Other recipes with concentration variations are available in the literature. Store stock solutions at 4 °C for up to one month.
- Salt solution: add 75.39 g of NaCl (129 mM final); 2.5 g of KCl (3.35 mM final); 0.81 g of NaH2PO4 (0.58 mM final); 2.33 g of MgCl2 (1.15 mM final); 1.85 g of CaCl2 (1.26 mM). Dissolve in 800 ml of distilled water then fill to 1 L with distillated water.
- Bicarbonate solution: add 17.65 g of NaHCO3 (21 mM final). Dissolve in 900 ml of distillated water then fill to 1 L with distilled water. Variations of the bicarbonate concentration will result in pH variations.
- Glucose solution: add 54.06 g of glucose (30 mM final). Dissolve in 400 ml of distillated water then fill to 500 ml with distilled water.
- Prepare aCSF by diluting 100 ml of salt solution, 100 ml of bicarbonate solution, and 50 ml of glucose solution in 1 L of distilled water. Store at 4 °C until use.
- Prepare a glass electrode by warming and stretching a glass tube until it breaks. Sand down the tip before use. The electrode can be reused many time as long as it stays clean.
- Prepare artificial cerebrospinal fluid (aCSF) stock solutions according to the following recipes. Other recipes with concentration variations are available in the literature. Store stock solutions at 4 °C for up to one month.
- Experimental Setup
NOTE: The set-up details are presented in Figure 1.- Turn on the amplifier, moving averager, data acquisition system, temperature controller and pump. Check the signal amplification (gain = 10,000), the filtration (low threshold, 10 Hz; high threshold, 5 kHz), and the sampling rate of the analog to digital conversion of the raw signal (2.5 kHz).
- Fill a bottle with aCSF and bubble it with carbogen (95% O2, 5% CO2). Induce a bubbled- and warmed-aCSF flow in the recording chamber (volume 5 ml) at 4 ml/min. Hold the temperature in the recording chamber at 26 (± 1) °C. pH should be 7.4 in these standard conditions.
- Keep 50 ml of room temperature (RT) and carbogen-bubbled aCSF in a 50 ml syringe near the dissection chamber.
- Turn on the computer and start the recording software.
2. Dissection
- Weigh and visually determine the sex of the animal (males have a longer ano-genital distance, while females have a short ano-genital distance; male genitals are often dark while female genitals are pink).
- Anesthetize the newborn rodents by one of the following options: cryoanesthesia (completely immerse the animal in ice for 4 – 5 min), injection (equitensine at 4 ml/kg) or inhalation of volatile anesthetics (isoflurane or ether). Confirm an adequate plane of anesthesia by the absence of a paw withdrawal reflex.
- Place the animal on the bench, ventral face down. Section the rostral part of the head coronally with a scalpel at the bregma level (visible through the skin and skull). Perform this step immediately after the animal is anesthetized.
- Section the body coronally with a scalpel under the anterior members.
- Remove skin, muscles, fatty tissues and viscus with surgical and thin scissors and pliers. Since bones are soft at this age, be cautious not to damage the nervous system. Perform this step on the bench.
- Place the preparation in the dissection chamber. Use the aCSF stored in the syringe to oxygenate the preparation. From this point to the end of the recording, use a microscope.
- On the dorsal face of the preparation, cut the skull and vertebras from the rostral to the caudal part along the medium axis with surgical and thin scissors and pliers. Open the cut skull and vertebras in order to expose the nervous tissue. Use the aCSF stored in the syringe to oxygenate the preparation.
- With the pliers, remove the arachnoid membrane, a thin tissue covering the surface of the nervous tissue. Retain the pia mater and blood vessels against the nervous tissue. Use the aCSF stored in the syringe to oxygenate the preparation.
- Place the preparation with the dorsal face down, and carefully bring down the brainstem and spinal cord by cutting the nerves and connective tissue with the scissors while holding the preparation in place. A dorsal approach is also possible. Keep the roots and nerves as long as possible. Remove the bones to isolate the brainstem and spinal cord. Use the aCSF stored in the syringe to oxygenate the preparation.
- Remove the cerebellum and remaining rostral structures by sectioning them with the scalpel. Use the aCSF stored in the needle to oxygenate the preparation.
- Optionally depending on experimental design, remove the pons with the scalpel by a coronal section anterior to the inferior cerebellar artery. Note that keeping the whole pons will slow down the rhythm in rats and fully inhibit it in mice. Preparations that include only the caudal part of the pons display a respiratory-like activity with a stable frequency at the C4 root.
3. Recording
- Place the preparation in the recording chamber, ventral face up. Fix the preparation with pins in the lowest part of the spinal cord and rostral-most part of the brainstem.
- Using a syringe linked to the electrode by its needle, induce a depression in the electrode (glass tip diameter: 150 - 225 µm) by pulling out the syringe piston, in order to partially fill the electrode with aCSF.
- Using a micromanipulator, carefully place the electrode close to one of the fourth ventral roots. Other ventral roots could also present a respiratory-like activity (e.g., XII cranial nerve, C1 ventral root).
- Induce a depression in the electrode tank via the syringe by pulling out the piston in order to gently aspirate the nerve rootlet. Then carefully move the electrode to apply it against the spinal cord.
NOTE: Ideally the size of the rootlet matches the size of the electrode opening and thus creates a seal between the internal and external compartments of the electrode. Since differential amplifiers are commonly used for such recordings, any opening between the inside of the electrode and the recording chamber reduces the quality of the signal and makes it difficult to eliminate background noise. - Start the recording.
- Record the rhythm produced by the preparation under normoxic conditions (i.e., aCSF bubbled with carbogen: 95% O2 and 5% CO2) for at least 20 min to determine the baseline parameters of the preparation.
- Switch the perfusion from carbogen-bubbled aCSF to stimulus aCSF (i.e., bubbled with 95% N2 and 5% CO2 for hypoxia stimulus) for 15 min. Alter exposure duration depending of the experimental protocol. Record the exposure duration on the recording and animal datasheet. Use stainless steel tubes between the aCSF bottle and the chamber whenever possible to avoid gas diffusion to the outside of the tube.
- Switch the perfusion back to standard carbogen-bubbled aCSF for at least 15 min for a recovery recording. Record this on the recording and animal datasheet.
- End the recording.
Wyniki
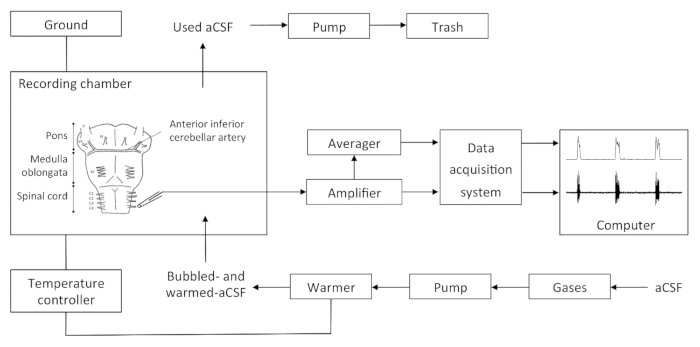
Figure 1. Recapitulative Schema of the Setup. The bubbled aCSF is warmed and sent by a pump in the recording chamber. aCSF excess in the recording chamber is aspirated by a pump and disposed of. The recording chamber is linked to the ground and the temperature controller. The electrode output is directly relayed to the amplifier, then to the moving averager and the data acquisition syst...
Ujawnienia
Materiały
| Name | Company | Catalog Number | Comments |
| Sylgard | Sigma Aldrich | 761036-5EA | Use under hood |
| NaCl | Bioshop | SOD002 | |
| KCl | Bioshop | POC888 | |
| CaCl2 | Bioshop | CCL444 | |
| MgCl2 | Bioshop | MAG510 | |
| NaHCO3 | Bioshop | SOB999 | |
| NaH2PO4 | Bioshop | SPM306 | |
| D-glucose | Bioshop | GLU501 | |
| Carbogen | Linde | 343-02-0006 | |
| Temperature Controller | Warner Instruments, Hamden, CT, USA | TC-324B | |
| Suction electrode | A-M Systems, Everett, WA, USA | model 573000 | |
| Differential AC amplifier | A-M Systems, Everett, WA, USA | model 1700 | |
| Moving averager | CWE, Ardmore, PA, USA | model MA-821 | |
| Data acquisition system | Dataq Instruments, Akron, OH, USA | model DI-720 | |
| LabChart software | ADInstruments, Colorado Springs, CO, USA | ||
| Prism sofware | Graphpad, La Jolla, CA, USA | ||
| Recording chamber | Home made | ||
| Base | Kanetec, Bensenville, IL, USA | ||
| Micromanipulator | World Precision Instrument Inc, Sarasota, FL, USA | MB | |
| Base | Kanetec, Bensenville, IL, USA | KITE-R | |
| Peristaltic pump | Gilson, Middleton, WI, USA | MB | |
| Faraday Cage | Home made | MINIPULS 3 | |
| Computer |
This article has been published
Video Coming Soon
Source: Rousseau, J., et al. Electrophysiology on Isolated Brainstem-spinal Cord Preparations from Newborn Rodents Allows Neural Respiratory Network Output Recording. J. Vis. Exp. (2015).
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone