Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Assessing Replication and Beta Cell Function in Adenovirally-transduced Isolated Rodent Islets
W tym Artykule
Podsumowanie
This protocol allows one to identify factors that modulate functional beta cell mass to find potential therapeutic targets for the treatment of diabetes. The protocol consists of a streamlined method to assess islet replication and beta cell function in isolated rat islets following manipulation of gene expression with adenoviruses.
Streszczenie
Glucose homeostasis is primarily controlled by the endocrine hormones insulin and glucagon, secreted from the pancreatic beta and alpha cells, respectively. Functional beta cell mass is determined by the anatomical beta cell mass as well as the ability of the beta cells to respond to a nutrient load. A loss of functional beta cell mass is central to both major forms of diabetes 1-3. Whereas the declining functional beta cell mass results from an autoimmune attack in type 1 diabetes, in type 2 diabetes, this decrement develops from both an inability of beta cells to secrete insulin appropriately and the destruction of beta cells from a cadre of mechanisms. Thus, efforts to restore functional beta cell mass are paramount to the better treatment of and potential cures for diabetes.
Efforts are underway to identify molecular pathways that can be exploited to stimulate the replication and enhance the function of beta cells. Ideally, therapeutic targets would improve both beta cell growth and function. Perhaps more important though is to identify whether a strategy that stimulates beta cell growth comes at the cost of impairing beta cell function (such as with some oncogenes) and vice versa.
By systematically suppressing or overexpressing the expression of target genes in isolated rat islets, one can identify potential therapeutic targets for increasing functional beta cell mass 4-6. Adenoviral vectors can be employed to efficiently overexpress or knockdown proteins in isolated rat islets 4,7-15. Here, we present a method to manipulate gene expression utilizing adenoviral transduction and assess islet replication and beta cell function in isolated rat islets (Figure 1). This method has been used previously to identify novel targets that modulate beta cell replication or function 5,6,8,9,16,17.
Protokół
1. Adenoviral Transduction and Culturing of Rat Islets
- Prepare a 6-well non-tissue culture coated plate by adding 2 ml of media (RPMI 1640 media containing 8 mM glucose, 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin) to the required number of wells. For example, a typical experiment may require three wells – one each for a no-virus control, a virus control (e.g., GFP-expressing adenovirus), and the experimental group.
- Warm the plate to 37 °C by placing it into a tissue culture incubator for at least 30 min.
- Immediately following rat islet isolation 18,19, place 100-200 islets into individual wells of the 6-well non-tissue culture coated plate. Sixty islets are required for the insulin secretion and thymidine incorporation assays. The remaining islets can be used for RNA isolation for gene expression studies or protein isolation for immunoblotting.
[Note: From this point forward, please follow institutional protocols for the handling, use, and disposal of biohazardous materials.]
- Gently swirl the plate to bring the islets to the center of the well.
- Pipette the adenovirus directly onto the islets in the center of the dish. Use 100-500 multiplicities of infection (MOI, the ratio of target cells to viral plaque-forming units).
- Let the islets rest for 5 min.
- Place the plate in the tissue culture incubator (37 °C, 5% CO2).
- After 24 h, gently swirl the plate to bring the islets to the centers of the wells and transfer the islets using a P200 micropipette to a new well containing fresh media. If the islets become attached to the plate, they can be gently dislodged with the pipette tip.
[Note: To verify adequate transduction efficiency, the use of a control virus expressing GFP is beneficial, as islets can then be imaged via confocal microscopy to verify penetration of the adenovirus into the islet core.]
- Culture the islets for an additional 24-72 h, depending on the desired timing of the experiment from optimization pilot studies. For example, induction of a proliferative response may require times ranging from 24-72 h or knockdown of the gene of interest may require 48 or 72 hours. Transfer the islets to fresh media each day.
- For the final 24 h of the experiment, culture the islets in media containing 1 μCi [methyl-3H]-thymidine/ml media (generally 1 μl thymidine/ml media).
[Note: From this point forward, please follow institutional protocols for the handling, use, and disposal of radioactive materials.]
2. Insulin Secretion Assay
- Prepare the secretion assay buffer (SAB) 10X stock solution (1.14 M NaCl, 47 mM KCl, 12 mM KH2PO4, 11.6 mM MgSO4) and CaCl2 100X stock solution (0.25 M CaCl2). These stock solutions may be prepared ahead of time and stored at room temperature.
- Freshly prepare 50 ml of the working SAB (5 mL of 10X SAB, 1 ml of 1 M HEPES, 0.5 ml of 100X CaCl2, 0.28 ml of 35% BSA, 0.11 g NaHCO3, and sterile water to 50 ml) in a 50-ml conical tube and warm to 37 °C by placing in a 37 °C waterbath.
- Pipette 10 ml of the working SAB into a 15-ml conical tube and add 66.8 μl of 2.5 M D-glucose to prepare the high glucose (16.7 mM) SAB.
- Add 44.8 μl of 2.5 M D-glucose to the remaining 40 ml of the working SAB to prepare the low glucose (2.8 mM) SAB.
- Label three 1.7-ml microcentrifuge tubes for each well of the 6-well plate and add 1 ml of phosphate-buffered saline (PBS).
[Note: As the islets are radioactive, please follow institutional protocols for the handling, use, and disposal of radioactive materials.]
- Place 20 islets into each microcentrifuge tube. Make every attempt to add comparably sized islets to each microcentrifuge tube. For example, each tube may contain 5 small-, 10 medium-, and 5 large-sized islets (see Figure 1).
[Note: Islets can be visualized using either a dissecting stereoscope or a standard microscope.]
- After the islets have settled on the bottom of the tube by gravity (~2 min), aspirate the PBS with a micropipette and discard.
[Note: As an alternative to settling by gravity, the tubes may be centrifuged at 300 x g for 1 min.]
- For pre-incubation, add 400 μl of the low glucose SAB, place the tubes (with their caps open) into the tissue culture incubator (37 °C, 5% CO2), and pre-incubate for 60 min. Aspirate the pre-incubation low glucose SAB and discard.
- For basal insulin secretion, add 400 μl of the low glucose SAB, place the tubes (with their caps open) into the tissue culture incubator (37 °C, 5% CO2), and incubate for 60 min. Collect the low glucose SAB and save for the insulin radioimmunoassay.
- For stimulated insulin secretion, add 400 μl of the high glucose SAB, place the tubes (with their caps open) into the tissue culture incubator (37 °C, 5% CO2), and incubate for 60 min. Collect the high glucose SAB and save for the insulin radioimmunoassay.
3. Thymidine Incorporation Assay
- Add 1 ml PBS; after the islets have settled on the bottom of the tube by gravity, aspirate the PBS with a micropipette, discard, and repeat this step once.
- Add 500 μl ice cold trichloroacetic acid (TCA, 10% w/v) and incubate on ice for 30 min.
- Centrifuge the tubes at 16 000 x g for 3 min at 4 °C.
- Aspirate the TCA, add 80 μl of 0.3 N NaOH, and incubate for 30 min at room temperature. During this time, vigorously vortex the samples for 5-10 s every 10 min.
- Add 4 ml of Econo-safe counting cocktail to 7 ml liquid scintillation counting tubes.
- Add 50 μl of the sample to the scintillation counting tube, cap the tube, shake briefly, and count in a liquid scintillation counter.
- Measure the protein concentration using the bicinchoninic acid (BCA) assay and 10 μl of sample according to the manufacturer's protocol.
4. Data Analysis
- Perform the insulin radioimmunoassay following the manufacturer's protocol.
- Normalize the insulin secretion and thymidine incorporation data with the protein concentration.
5. Representative Results
An example of the experiment to assess islet replication and beta cell function in rat islets is shown in Figure 2. This example shows that adenoviral overexpression of hypothetical "Gene #6" robustly stimulates islet replication without altering beta cell function. In the top panel, the results from the thymidine incorporation assay demonstrate that increasing the expression of "Gene #6" increases DNA synthesis, as measured by the incorporation of thymidine. Because most of the cells in the rat islet are beta cells, it is likely that this increase in thymidine incorporation indicates an increase in beta cell replication. However, confirmatory experiments must be performed to firmly establish this. In the bottom panel, the results from the insulin secretion assay demonstrate that overexpression of "Gene #6" did not alter one of the primary beta cell functions, i.e., insulin secretion at low and high glucose. The quality of the islet isolation and health of the islets following treatment with adenoviruses is indicated by the fold increase in insulin secretion at low and high glucose concentrations. If increasing the expression of "Gene #6" impaired beta cell function, this would likely be reflected as a decrease in the insulin secreted at high, stimulatory glucose concentrations (16.7 mM). A dose-response curve for varying glucose concentrations could also be performed.
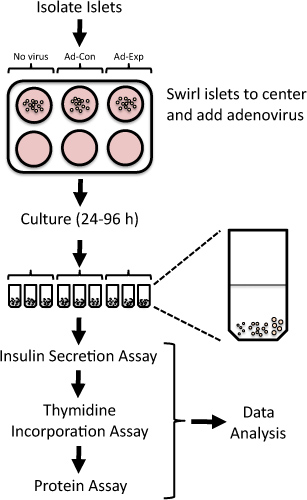
Figure 1. Overview of protocol to assess islet replication and beta cell function in isolated rat islets following adenoviral-mediated alterations in gene expression. Freshly isolated rat islets are exposed to adenoviruses for 24 h and then cultured up to 96 h. Thymidine incorporation is assessed in the final 24 h, followed by the measurement of insulin secretion at low and high glucose.
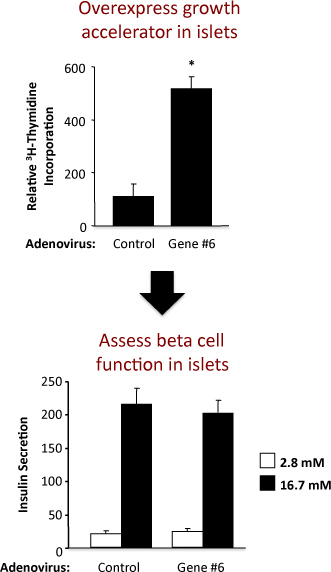
Figure 2. Results from an experiment using a control adenovirus and an adenovirus overexpressing a hypothetical gene labeled as "Gene #6". The top panel shows the thymidine incorporation and the bottom panel the insulin secretion.
Dyskusje
Establishing pathways that can be modulated to stimulate the replication and enhance the function of beta cells are relevant to both major forms of diabetes. Because functional beta cell mass is dependent on the existence and function of insulin-secreting cells, assessing these determinants simultaneously has its advantages. This protocol describes a streamlined protocol for identifying whether overexpression or suppression of a protein leads to changes in functional beta cell mass in vitro, which can the...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was supported by grant DK078732 from the NIH (to P.T.F).
Materiały
| Name | Company | Catalog Number | Comments |
| RPMI 1640 media | Gibco | 11879 | |
| Penicillin/streptomycin | Gibco | 15140 | |
| 6-well plate | BD-Falcon | 35-1146 | Non-TC treated |
| [methyl-3H]-thymidine | Perkin Elmer | NET027Z001MC | 1 mCi/ml |
| Micro-centrifuge tubes | Denville | C2170 | 1.7 ml |
| NaCl | Sigma | 59888 | |
| KCl | Acros | 42409 | |
| KH2PO4 | Acros | 20592 | |
| MgSO4 | Acros | 41348 | |
| CaCl2 | Acros | 34961 | |
| HEPES | Sigma | H0887 | 1 M solution |
| 35% BSA | Sigma | A7979 | |
| NaHCO3 | Acros | 42427 | |
| d-glucose | Sigma | G8769 | |
| TCA | Fisher Scientific | SA9410-1 | 10% w/v |
| NaOH | Acros | 12426 | |
| Scintillation counting tube | Sarstedt | 58.536 | 7 ml, PP |
| Scintillation counting tube cap | Sarstedt | 65.816 | |
| Econo-Safe counting cocktail | RPI | 111175 | |
| Insulin RIA | Siemens | TKIN2 | |
| BCA Assay Kit | Thermo Scientific | 23250 | |
| Equipment | |||
| Centrifuge | Eppendorf | 5415R | |
| Scintillation counting tube rack | Sarstedt | 93.1431.001 | |
| Liquid scintillation counter | Perkin Elmer | Tri-Carb 2910TR | |
Odniesienia
- Ferrannini, E. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J. Clin. Endocrinol. Metab. 90, 493-500 (2005).
- Weyer, C., Bogardus, C., Mott, D. M., Pratley, R. E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest. 104, 787-794 (1999).
- Keenan, H. A. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 59, 2846-2853 (2010).
- Bain, J. R., Schisler, J. C., Takeuchi, K., Newgard, C. B., Becker, T. C. An adenovirus vector for efficient RNA interference-mediated suppression of target genes in insulinoma cells and pancreatic islets of langerhans. Diabetes. 53, 2190-2194 (2004).
- Fueger, P. T. Trefoil factor 3 stimulates human and rodent pancreatic islet beta-cell replication with retention of function. Mol. Endocrinol. 22, 1251-1259 (2008).
- Schisler, J. C. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol. Cell Biol. 28, 3465-3476 (2008).
- Chan, C. B. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 48, 1482-1486 (1999).
- Cozar-Castellano, I., Takane, K. K., Bottino, R., Balamurugan, A. N., Stewart, A. F. Induction of beta-cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenovirus-mediated transfer of cyclin-dependent kinase-4 and cyclin D1. Diabetes. 53, 149-159 (2004).
- Icyuz, M. Adenovirus infection activates akt1 and induces cell proliferation in pancreatic islets1. Transplantation. 87, 821-824 (2009).
- Kaneto, H. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J. Biol. Chem. 276, 31099-31104 (2001).
- Antinozzi, P. A., Berman, H. K., O'Doherty, R. M., Newgard, C. B. Metabolic engineering with recombinant adenoviruses. Annu. Rev. Nutr. 19, 511-544 (1999).
- Newgard, C. B., Becker, T. C., Berman, H. K., O'Doherty, R. M. Regulation of overexpressed hexokinases in liver and islet cells. Biochem. Soc. Trans. 25, 118-122 (1997).
- Becker, T. C., BeltrandelRio, H., Noel, R. J., Johnson, J. H., Newgard, C. B. Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus. Enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J. Biol. Chem. 269, 21234-21238 (1994).
- Csete, M. E. Adenoviral-mediated gene transfer to pancreatic islets does not alter islet function. Transplant Proc. 26, 756-757 (1994).
- Csete, M. E. Efficient gene transfer to pancreatic islets mediated by adenoviral vectors. Transplantation. 59, 263-268 (1995).
- Meng, Z. X. Activation of liver X receptors inhibits pancreatic islet beta cell proliferation through cell cycle arrest. Diabetologia. 52, 125-135 (2009).
- Ronnebaum, S. M. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J. Biol. Chem. , (2006).
- Milburn, J. L. Pancreatic beta-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J. Biol. Chem. 270, 1295-1299 (1995).
- Szot, G., Koudria, P., Bluestone, J. Murine Pancreatic Islet Isolation. J. Vis. Exp. (7), e255 (2007).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone