Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Metal-silicate Partitioning at High Pressure and Temperature: Experimental Methods and a Protocol to Suppress Highly Siderophile Element Inclusions
W tym Artykule
Podsumowanie
We present a procedure to determine the metal-silicate partitioning of siderophile elements, emphasizing techniques that suppress the formation of metal inclusions in experiments for the noble metals. The results of these experiments are used to demonstrate the effect of core-formation on the highly siderophile element composition of the mantle.
Streszczenie
Estimates of the primitive upper mantle (PUM) composition reveal a depletion in many of the siderophile (iron-loving) elements, thought to result from their extraction to the core during terrestrial accretion. Experiments to investigate the partitioning of these elements between metal and silicate melts suggest that the PUM composition is best matched if metal-silicate equilibrium occurred at high pressures and temperatures, in a deep magma ocean environment. The behavior of the most highly siderophile elements (HSEs) during this process however, has remained enigmatic. Silicate run-products from HSE solubility experiments are commonly contaminated by dispersed metal inclusions that hinder the measurement of element concentrations in the melt. The resulting uncertainty over the true solubility and metal-silicate partitioning of these elements has made it difficult to predict their expected depletion in PUM. Recently, several studies have employed changes to the experimental design used for high pressure and temperature solubility experiments in order to suppress the formation of metal inclusions. The addition of Au (Re, Os, Ir, Ru experiments) or elemental Si (Pt experiments) to the sample acts to alter either the geometry or rate of sample reduction respectively, in order to avoid transient metal oversaturation of the silicate melt. This contribution outlines procedures for using the piston-cylinder and multi-anvil apparatus to conduct solubility and metal-silicate partitioning experiments respectively. A protocol is also described for the synthesis of uncontaminated run-products from HSE solubility experiments in which the oxygen fugacity is similar to that during terrestrial core-formation. Time-resolved LA-ICP-MS spectra are presented as evidence for the absence of metal-inclusions in run-products from earlier studies, and also confirm that the technique may be extended to investigate Ru. Examples are also given of how these data may be applied.
Wprowadzenie
Terrestrial accretion is thought to have occurred as a series of collisions between planetesimals with a chondritic bulk composition, terminating in a giant-impact phase thought responsible for moon formation1,2. Heating of the proto-earth by impacts and the decay of short-lived isotopes was sufficient to cause extensive melting and the formation of a magma ocean or ponds through which dense Fe-rich metallic melts could descend. Upon reaching the base of the magma ocean, metallic melts encounter a rheological boundary, stall, and undergo final metal-silicate equilibrium before eventually descending through the solid mantle to the growing core2. Further chemical communication between metal and silicate phases as metallic melt traverses the solid portion of the mantle is thought to be precluded due to the large size and rapid descent of metal diapirs3. This primary differentiation of the Earth into a metallic core and silicate mantle is revealed today by both geophysical and geochemical observations4–6. Interpreting these observations to yield plausible conditions for metal-silicate equilibrium at the base of a magma ocean, however, requires an appropriate database of experimental results.
The primitive upper mantle (PUM) is a hypothetical reservoir comprising the silicate residue of core formation and its composition therefore reflects the behaviour of trace elements during metal-silicate equilibrium. Trace elements are distributed between metal and silicate melts during core segregation on the basis of their geochemical affinity. The magnitude of an elements preference for the metal phase can be described by the metal-silicate partition coefficient 
 (1)
(1)
Where  and
and 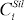 denote the concentration of element i in metal and silicate melt respectively. Values of
denote the concentration of element i in metal and silicate melt respectively. Values of 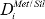 >1 indicate siderophile (iron-loving) behaviour and those <1 lithophile (rock-loving) behavior. Estimates of the PUM composition show that siderophile elements are depleted relative to chondrites7, typically considered as representative of Earth’s bulk composition6,8. This depletion is due to sequestration of siderophile elements by the core, and for refractory elements its magnitude should directly reflect values of
>1 indicate siderophile (iron-loving) behaviour and those <1 lithophile (rock-loving) behavior. Estimates of the PUM composition show that siderophile elements are depleted relative to chondrites7, typically considered as representative of Earth’s bulk composition6,8. This depletion is due to sequestration of siderophile elements by the core, and for refractory elements its magnitude should directly reflect values of 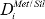 . Lab experiments therefore seek to determine values of
. Lab experiments therefore seek to determine values of 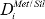 over a range of pressure (P), temperature (T) and oxygen fugacity (fO2) conditions that are relevant to metal segregation from the base of a magma ocean. The results of these experiments may then be used to delineate regions of P-T-fO2 space that are compatible with the PUM abundance of multiple siderophile elements (e.g.,9–11).
over a range of pressure (P), temperature (T) and oxygen fugacity (fO2) conditions that are relevant to metal segregation from the base of a magma ocean. The results of these experiments may then be used to delineate regions of P-T-fO2 space that are compatible with the PUM abundance of multiple siderophile elements (e.g.,9–11).
The high pressures and temperatures relevant to a magma ocean scenario can be recreated in the laboratory using either a piston-cylinder or multi-anvil press. The piston-cylinder apparatus provides access to moderate pressure (~2 GPa) and high temperature (~2,573 K) conditions, but enables large sample volumes and a variety of capsule materials to be easily used. The rapid cooling rate also permits quenching of a range of silicate melt compositions to a glass, thus simplifying textural interpretation of the run-products. The multi-anvil apparatus typically employs smaller sample volumes but with suitable assembly designs can achieve pressures up to ~27 GPa and temperatures of ~3,000 K. The use of these methods has allowed partitioning data for many of the moderately and slightly siderophile elements to be gathered over a large range of P-T conditions. Predictions of the PUM composition based on these data suggest metal-silicate equilibrium occurred at average pressure and temperature conditions in excess of ~29 GPa and 3,000 K respectively, although the exact values are model dependent. In order to account for the PUM abundance of certain redox sensitive elements (e.g., V, Cr) the fO2 is also thought to evolve during accretion from ~4 to 2 log units below that imposed by co-existing iron and wüstite (FeO) at equivalent P-T conditions (the iron-wüstite buffer)12.
Although the PUM abundance of many siderophile elements can be accounted for by metal-silicate equilibrium at the base of a deep magma ocean, it has proved difficult to assess if this situation also applies to the most highly siderophile elements (HSEs). The extreme affinity of the HSEs for iron-metal indicated by low pressure (P ~0.1 MPa) and temperature (T <1,673 K) experiments suggests the silicate earth should be strongly depleted in these elements. Estimates of the HSE content for PUM, however, indicate only a moderate depletion relative to chondrite (Figure 1). A commonly posited solution to the apparent HSE excess is that Earth experienced a late-accretion of chondritic material subsequent to core-formation13. This late-accreted material would have mixed with the PUM and elevated HSE concentrations but had a negligible effect on more abundant elements. Alternatively, it has been suggested that the extremely siderophile nature of HSEs indicated by low P-T experiments does not persist to the high P-T conditions present during core-formation14,15. In order to test these hypotheses, experiments must be carried out to determine the solubility and metal-silicate partitioning of HSEs at appropriate conditions. Contamination of the silicate portion of quenched run-products in many previous studies however, has complicated run-product analysis and obscured the true partition coefficients for HSEs between metal and silicate melts.
In partitioning experiments where the HSEs are present at concentration levels appropriate to nature, the extreme preference of these elements for Fe-metal prevents their measurement in the silicate melt. To circumvent this problem, solubility measurements are made in which the silicate melt is saturated in the HSE of interest and values of 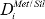 are calculated using the formalism of Borisov et al.16. Quenched silicate run-products from HSE solubility experiments performed at reducing conditions, however, often display evidence for contamination by dispersed HSE±Fe inclusions17. Despite the near ubiquity of these inclusions in low fO2 experiments containing Pt, Ir, Os, Re and Ru, (e.g., 18–27), there is notable variability between studies in their textural presentation; compare for example references 22 and 26. Although it has been demonstrated that inclusions can form which are a stable phase at the run conditions of an experiment28, this does not preclude the formation of inclusions as the sample is quenched. Uncertainty surrounding the origin of inclusions makes the treatment of analytical results difficult, and has led to ambiguity over the true solubility of HSEs in reduced silicate melts. Inclusion-free run-products are required to assess which studies have adopted an analytical approach that yields accurate dissolved HSE concentrations. Considerable progress in suppressing the formation of metal-inclusions at reducing conditions has now been demonstrated in experiments using a piston-cylinder apparatus, in which the sample design was amended from previous studies by adding either Au or Si to the starting materials29–31. The addition of Au or elemental Si to the starting materials alters the sample geometry or fO2 evolution of the experiment respectively. These methods are intended to suppress metal inclusion formation by altering the timing of HSE in-diffusion versus sample reduction, and are discussed in Bennett et al.31. Unlike some previous attempts to cleanse the silicate melt of inclusions, such as mechanically assisted equilibration and the centrifuging piston-cylinder, the present protocol can be implemented without specialized apparatus and is suitable for high P-T experiments.
are calculated using the formalism of Borisov et al.16. Quenched silicate run-products from HSE solubility experiments performed at reducing conditions, however, often display evidence for contamination by dispersed HSE±Fe inclusions17. Despite the near ubiquity of these inclusions in low fO2 experiments containing Pt, Ir, Os, Re and Ru, (e.g., 18–27), there is notable variability between studies in their textural presentation; compare for example references 22 and 26. Although it has been demonstrated that inclusions can form which are a stable phase at the run conditions of an experiment28, this does not preclude the formation of inclusions as the sample is quenched. Uncertainty surrounding the origin of inclusions makes the treatment of analytical results difficult, and has led to ambiguity over the true solubility of HSEs in reduced silicate melts. Inclusion-free run-products are required to assess which studies have adopted an analytical approach that yields accurate dissolved HSE concentrations. Considerable progress in suppressing the formation of metal-inclusions at reducing conditions has now been demonstrated in experiments using a piston-cylinder apparatus, in which the sample design was amended from previous studies by adding either Au or Si to the starting materials29–31. The addition of Au or elemental Si to the starting materials alters the sample geometry or fO2 evolution of the experiment respectively. These methods are intended to suppress metal inclusion formation by altering the timing of HSE in-diffusion versus sample reduction, and are discussed in Bennett et al.31. Unlike some previous attempts to cleanse the silicate melt of inclusions, such as mechanically assisted equilibration and the centrifuging piston-cylinder, the present protocol can be implemented without specialized apparatus and is suitable for high P-T experiments.
Described in detail here is a piston-cylinder based approach to determine the solubility of Re, Os, Ir, Ru, Pt and Au in silicate melt at high temperature (>1,873 K), 2 GPa and an fO2 similar to that of the iron-wüstite buffer. Application of a similar experimental design may also prove successful in HSE experiments at other pressures, providing the required phase relations, wetting properties and kinetic relationships persist to the chosen conditions. Existing data however, are insufficient to predict whether our sample design will be successful at pressures corresponding to a deep magma ocean. Also outlined is a general approach used to determine moderately and slightly siderophile element (MSE and SSE respectively) partitioning using a multi-anvil device. Extension of the inclusion-free dataset for HSEs to high pressure is likely to employ similar multi-anvil methods. Together, these procedures provide a means to constrain both the conditions of core-segregation and the stages of terrestrial accretion.
Protokół
1) Preparation of Starting Material
- Synthetic Basalt
Note: A basaltic composition is used as the silicate starting material as more depolymerized compositions, although more relevant to a magma ocean scenario, are difficult or impossible to quench to a glass in piston-cylinder and multi-anvil experiments.- Weigh the desired amounts of component oxide or carbonate (Ca and Na) powders, with the exception of Fe, and add to an agate mortar (see example in Table 1). An Fe-free mixture weighing ~4 g should provide sufficient starting material for an extensive suite of experiments.
- Add ethanol to the agate mortar until the powders are submerged then grind for at least 2 hr using an agate pestle to homogenize both the composition and grain size of the mixture.
Note: Homogeneity of ground starting compositions can be checked by examining a pressed pellet of the powdered mixture with a scanning electron microscope equipped for compositional analysis by energy dispersive x-ray spectroscopy. - Once thoroughly homogenized, place the mortar under a 250 W heat lamp, at a distance of ~20 cm. After the powdered mixture is dry, which may take 20-60 min, transfer it to either an alumina or mullite (an alumino-silicate) crucible.
- To decarbonate the mixture, place the crucible with the powdered mixture into a box furnace at RT and ramp to 1,273 K over the course of 3-5 hr. Leave the mixture in the furnace at 1,273 K O/N.
- Remove the decarbonated mixture from the box furnace and allow it to cool to RT. Once cool, weigh and add iron to the mixture as either FeO or Fe2O3 powder (see Table 1). Varying the ratio of FeO to Fe2O3 while keeping the total Fe content the same allows the final fO2 of the sample to be changed. To access more reducing conditions, and in all experiments to investigate Pt, also add ~0.5-2.0 wt% Si to the mixture. Once Fe (±Si) has been added, re-homogenize the mixture by again grinding under ethanol with an agate mortar and pestle.
- Dry the homogenized mixture under a heat lamp and then transfer it to a shell vial. Store in a desiccator until ready to load the sample capsule.
- Metallic Phase: Re, Os, Ir, Ru Experiments
- For experiments intended to investigate Re, Os, Ir or Ru, prepare a 3:1 by weight (6:1 for Ru, to account for the difference in atomic mass) mixture of Au and the HSE of interest, using high purity metallic powders. A mixture weighing ~500 mg should provide sufficient starting material for an extensive suite of experiments.
- Transfer the mixture into a graphite crucible and cover with a graphite lid. Then place the covered crucible into a box furnace at a temperature of 1,473 K for ~5 min. Once removed from the furnace, leave the crucible lid in place until the assembly has cooled to RT.
CAUTION: Heating of osmium in air may result in formation of the toxic compound osmium tetroxide. Osmium metal is also a known skin irritant, see MSDS for CAS# 7440-04-2.
Note: This process melts the Au (melting point ~1,337 K) but not the accompanying HSE, resulting in formation of a metallic bead where the HSE of interest is surrounded by a rind of Au. - Remove the metallic bead from the graphite crucible and use a razor blade to divide it into smaller pieces which measure ~1 mm in their longest dimension. Once cut, place the beads into a shell vial and store in a desiccator.
- Metallic Phase: Pt Experiments
Note: Experiments to investigate Pt cannot be performed using the Au-coated bead technique due to the complete miscibility of Pt and Au at high temperature (>2,042 K at 0.1 MPa32). This precludes a sample geometry whereby Pt is physically separated from the silicate melt during an experiment by a rind of Au.- Thoroughly mix metallic powders of Pt and Ir in a 1:1 ratio by weight to make a total of ~500 mg of mixture. Next, add ~20 mg of metallic Fe powder so that Fe comprises ~4 weight percent of the total mixture.
- Tape a clean drill blank (alternatively, the shank of a drill may be used in place of a drill blank) to the edge of a workbench so that ~3 mm protrudes from the tabletop. Position a silica glass tube, with an internal diameter of ~2-3 mm and external diameter of ~4-6 mm, onto the protruding end of the drill blank.
- Place the PtIrFe mixture into the glass tube and insert another drill blank above it. Both drill blanks should have a diameter not more than 0.1 mm smaller than the inner diameter of the silica glass tube. Cold-press the metallic mixture by pushing the drill blanks together by hand (Figure 2).
CAUTION: Use of excessive force during the cold-pressing step can cause the silica glass to shatter). - Put the cold-pressed powders, still inside the silica glass tube, into an alumina crucible and suspend in the cool portion of a gas-mixing vertical tube furnace. Increase the furnace temperature to 1,673 K and using CO-CO2 gas mixtures, set the furnace fO2 to a value close to the iron-wüstite buffer.
Note: At ambient pressure and 1,673 K, the iron-wüstite buffer corresponds to a fO2 of 1.93 x 10-10 Pa33. The relationship between CO-CO2 mixing ratio, temperature and fO2 can be found in reference 34. For the iron-wüstite buffer at 1,673 K use a gas mixture comprising 22.25 vol% CO2 and 77.75 vol% CO.- Once the desired temperature and fO2 are reached, lower the alumina crucible so that it resides in the furnace hot spot and leave O/N for the pressed powders to anneal.
- Remove the crucible and pressed powders from the gas-mixing furnace and allow them to cool. If the silica glass tube is still intact, use a drill blank to push the annealed powder out of the tube. Using wire cutters, break the annealed powder into pieces small enough to fit within the sample capsule chosen for the experiment.
- Transfer the metallic pieces to a shell vial and store in a desiccator until required.
- Metallic Phase: Multi-anvil Experiments
- For experiments to determine the partitioning of moderately and slightly siderophile elements, mix the synthetic basalt powder with Fe-metal powder in equal proportions.
Note: Some portion of the Fe may be added as a Fe-Si alloy, typically so that Si comprises <8 wt% of the metallic fraction. This will ensure the experimental fO2 remains low. - Add the chosen trace elements as metal-oxide powders to the basalt plus metal mixture. Homogenize the starting material by grinding under ethanol with an agate mortar and pestle. The exact amount of trace elements added will depend upon the element being investigated, however, nominal concentrations of several thousand ppm to 2 wt% are typical10,35.
- Once homogenized, dry the powdered starting material under a heat lamp, transfer it to a shell vial then store in a desiccator until needed.
- For experiments to determine the partitioning of moderately and slightly siderophile elements, mix the synthetic basalt powder with Fe-metal powder in equal proportions.
2. Preparation of Assembly Components
- Piston Cylinder
Note: The piston cylinder assembly consists of a graphite capsule that is supported in the hot spot of a graphite resistance heater using crushable magnesia pieces. An alumina sheathed thermocouple is positioned axially through the upper portion of the assembly to monitor temperature at the top of the sample. The furnace is then surrounded by BaCO3 cells which act as both a pressure medium and thermal insulator36. The assembly dimensions are provided in Figure 3A. A list of example materials used for the experiments and their sources are provided in Table 2.- Machine the graphite capsules, graphite end plug and magnesia support pieces to the required dimensions with a center lathe, using high purity graphite and magnesia rods or tubes respectively as starting materials (Figure 3A).
Note: For experiments to investigate Re, Os and Ir, HSE-Fe alloys may be substituted for graphite as the capsule material29,30. - Sonicate the graphite capsules in ethanol for ~1 min at RT, then dry under a heat lamp in the same manner as directed for powdered starting materials. Once dry, transfer the capsules to a shell vial and store in a desiccator or drying oven until required.
- Place the magnesia support pieces in either an alumina or mullite crucible and anneal at 1,573 K in a box furnace for at least 8 hr. After annealing, allow the pieces to cool then store in a drying oven maintained at ~393 K.
- To make the barium carbonate cells, first mix BaCO3 powder and used copy toner in 99:1 proportions by weight. A minimum of 7.4 grams of mixture is required for one experiment. Coat the interior portion of an appropriately sized steel die (see Figure 3A for dimensions of the BaCO3 sleeves) with either a graphite-based dry lubricant or PTFE-based mold release agent (Table 2).
- Cold-press 3.7 g of the mixture to ~250 MPa using the steel die and a hydraulic press. Leave the mixture at pressure for 1 min before decompression. This will produce a sleeve with a height of 17 mm. Two sleeves are required for each assembly.
Note: The 2-cell arrangement described above and used in some previous studies29–31 may be substituted for a single BaCO3 cell providing a suitably sized die is available. - Once removed from the die, drive-off the copy toner by heating the sleeves from RT to 923 K over the course of several hours in a box furnace, then holding at this temperature for ~30 min. Note the change in color from black to orange once the copy toner has been removed. Store the annealed sleeves in a drying oven maintained at ~393 K.
- Machine the graphite capsules, graphite end plug and magnesia support pieces to the required dimensions with a center lathe, using high purity graphite and magnesia rods or tubes respectively as starting materials (Figure 3A).
- Multi-anvil
Note: The multi-anvil assembly comprises a sample capsule that is positioned in the hot spot of a cylindrical graphite resistance heater using crushable MgO or Al2O3 filler pieces. The heater is surrounded by either a sintered or castable ceramic octahedron that acts as both a pressure medium and thermal insulator. The thermocouple may be positioned either axially or transversely depending upon the assembly design. There are numerous sizes and designs of assembly used for multi-anvil experiments, depending upon the desired objective and P-T conditions. Figure 4 displays an assembly design previously used to perform metal-silicate partitioning experiments at 3.6 and 7.7 GPa35.- Prepare graphite capsules and crushable magnesia or zirconia sleeves from high purity tubes in the same manner as indicated for piston cylinder experiments. The required dimensions are provided in Figure 4A.
- Make the alumina plug from a length of hard-fired alumina rod. Use a diamond file to score the rod where it is to be broken, then snap the rod to the required length by hand (see Figure 4A for dimensions). Use the file to remove any burrs that result from breaking the rod. Clean the plug by sonicating it in ethanol at RT.
- Prepare octahedra with an 18 mm octahedral edge length (OEL) using an MgO-based castable 2-part ceramic (see Table 2) and appropriately sized mold. The mold comprises a jig which holds 8 truncated cubes, separated by sheets with a thickness equal to that desired for the pre-formed gaskets37.
- For octahedra with an 18 mm OEL, use cubes with an 11 mm truncated edge length (TEL) and sheets that are 3 mm thick. Use either aluminum or PVC for the cube and sheet materials. Assemble the mold, lubricating all parts that will contact the castable ceramic with silicone grease. Leave one cube unassembled to provide an entry point for the ceramic mixture.
- Combine the powder ceramic and liquid activator in a 100:30 ratio by weight and mix thoroughly. Pour the mixture into the mold, ensuring there are no trapped pockets of air. Insert the remaining cube and allow the mixture to set for at least 2 hr. Each octahedron requires ~15 g of ceramic mixture.
- Once set, remove the octahedron from the mold, dehydrate for ~1 day in a drying oven at 393 K then anneal at 1,273-1,373 K in a box furnace for ~2 hr.
- Allow the octahedron to cool to RT in air, then drill a 7.3 mm diameter hole as indicated in Figure 4B to accommodate the insulating sleeve, graphite heater and remaining sample components.
- Store in a drying oven at ~393 K until ready to assemble the experiment.
3. Assembly of the Components
- Assembly of the Piston-cylinder Experiment
- Load the graphite sample capsule by first inserting the HSE-bearing metal then adding synthetic basalt powder until the capsule is filled. Use of a gravitationally stable arrangement minimizes the chance for overturn during the experiment and is intended to prevent dispersion of the metallic phase through mechanical action.
- Place a small amount (typically <50 mg) of dry MgO powder at the base of the cavity designed to hold the sample capsule. This flattens the tapered surface created when drilling the hole and in turn reduces shear forces during sample compression that may crack the capsule.
- Assemble all the previously made components as shown in Figure 3B.
- Wrap a piece of 30 µm thick lead foil around the assembly, folding a small (~1.5 mm) portion of foil over the exposed end of the lower BaCO3 sleeve. Insert the assembly into a 12.7 mm bore tungsten carbide pressure vessel, along with a base plug (above) and steel end-piece (below) as shown in Figure 3A.
Note: The end-loaded piston-cylinder apparatus has two hydraulic rams. A bridge straddling the lower ram allows a tungsten carbide piston to apply pressure to the bottom of the sample. The upper ram fixes the location of the upper sample surface and applies an end-load to the pressure vessel that gives additional support to the tungsten carbide core38. Figure 3C shows a piston cylinder apparatus at the University of Toronto with the bridge in place. A friction-correction of -9% is applied to account for the difference between the nominal sample pressure and that experienced by the sample39. - Position the bridge, pressure vessel and base-plate between the hydraulic rams. Next make a C-type thermocouple using 4-hole hard-fired alumina tube with an outer diameter of 1.6 mm. The alumina tube should be cut sufficiently long to allow ~1-2 mm of the tube to protrude from the upper surface of the top-plate.
- Feed both wire compositions (see Table 2) through adjacent holes in the tube, turn the ends through 180 degrees and secure them in the opposing holes so that the wires cross. Insert the thermocouple through the top plate and into the assembly, so that the junction is directly above the sample. Insulate the remainder of the thermocouple wires using flexible Teflon tubes, leaving a 10-20 mm portion exposed at the end.
- Place any required metal spacers in place between the top plate and the upper ram. During assembly, position Mylar sheets both above the pressure vessel and between the top of the assembly and the upper ram. These sheets electrically isolate the sample heating circuit from the rest of the apparatus.
- Assembly of the Multi-anvil Experiment
- Make a C-type thermocouple using 4-hole hard-fired alumina tube by feeding both wires through adjacent holes in the tube, turning the ends through 180° and securing them in the opposing holes. Insulate the remainder of the wires with a short length (~20 mm) of alumina tube and then Teflon insulating material, leaving a 10-20 mm portion of exposed wire at the end.
- Insert the zirconia sleeve and graphite heater into the octahedron, then cut grooves as indicated in Figure 4B. Insert the thermocouple into the top of the octahedron and position the alumina covered arms into the grooves. Use zirconia cement (see Table 2) to fill the void space surrounding the thermocouple and allow to dry.
- In order to isolate the thermocouple join from the graphite capsule, add MgO powder from the base of the octahedron until the exposed wires are covered. Less than 50 mg of powder are usually sufficient to surround the exposed wire. To ensure tight packing of the MgO powder, use a drill blank to tamp down the loose powder.
- Load a graphite capsule with the previously prepared sample material and place into the octahedron from the open side. Insert the alumina plug to complete assembly of the octahedron.
- On 4 of the WC cubes (Table 2) use polyvinyl acetate to glue short lengths of balsa-wood, one on each of the 3 faces adjacent to the truncated corner of the cube. Each balsa-wood piece should measure ~4.4 mm in height and width by ~9.0 mm in length, for the octahedron size shown in Figure 4. On each face, position the balsa-wood pieces in the quadrant opposite the truncated edge.
- Assemble 4 of the cubes to form a square in plane view, 2 with and 2 without wooden pieces attached. Orient the truncated edges to face the center of the square.
- Position the octahedron in the center of the cubes, so that it is supported by the truncated edges. Then angle the thermocouple arms so that they emerge from opposite corners of the square (Figure 5A)
- Place the remaining WC cubes into position to form a cube with the octahedron at its center, ensuring that the cubes with wooden pieces attached rest atop cubes that have no wooden spacers.
- Glue square pieces of ~0.5 mm thick G10 sheet (see Table 2) to each face of the assembled cube using a cyanoacrylate-type adhesive. For 32 mm WC cubes, use G10 sheets measuring ~55 mm x 55 mm. Two of the WC cubes have truncations that contact the resistance heater and thus form part of the electrical heating circuit. For sheets which contact these cubes, cut 2 narrow (<1 mm width) slits as indicated in Figure 5B and place a piece of copper foil so that it provides a point of contact between the 1st and 2nd-stage anvils.
Note: The multi-anvil apparatus utilizes a 2-stage system of anvils contained within a retaining ring. The first stage anvils comprise 6 removable wedges that form a central cubic cavity. This cavity accommodates 8 tungsten carbide cubes with truncated corners (second stage anvils) that surround the ceramic octahedron40. Vertically oriented force applied to the first stage anvils by a hydraulic press is therefore transferred to the octahedron in a manner that results in quasi-hydrostatic compression of the sample. The relationship between oil pressure in the ram and sample pressure can be calibrated for the 18 mm OEL cast octahedral assembly described here using the procedures outlined by41. - Cut 2 sheets of 0.076 mm thick Mylar to the dimensions shown in Figure 6 and coat them using a dry PTFE lubricant.
- Position one of the pre-cut sheets into the retaining ring (straight edge at the base) and insert the lower-set of 1st-stage anvils, which themselves are backed with 0.076 mm thick Mylar and coated with PTFE lubricant (Figure 5B). The lower set of anvils may be left in place between runs. Place the assembled cube into the lower set of 1st-stage anvils and connect the thermocouple arms to balanced thermocouple wires that exit the pressure module.
- Position the 2nd pre-cut Mylar sheet into the retaining ring (straight edge to the top) and insert the upper set of 1st-stage anvils, which should be Mylar backed and lubricated in the same manner as the lower set. This arrangement yields a lubricated Mylar to Mylar contact between the 1st-stage anvils and the retaining ring that reduces the loss of ram thrust to friction by ~30% compared to a single Mylar sheet arrangement37.
Note: Thicknesses and dimensions of the Mylar sheet will depend upon the exact design of the pressure module being used. Described above and in Figure 6 are the dimensions in use at the Geophysical Laboratory, Carnegie Institution of Washington.
4. Running the Experiment
- Once the sample is brought to the required pressure, heat at a rate of 100 K/min until the desired dwell temperature is reached. During the heating step, oil in the sample ram may need to be adjusted in order to maintain a constant oil pressure.
- After the dwell period, quench the sample by cutting power to the furnace. Once the apparatus has cooled to RT, slowly decompress the sample.
5. Run-product Analysis
- For piston cylinder experiments, extract the finished experiment from the pressure vessel using a hydraulic ram. With a pair of heavy-duty cutters remove the outer portions of the assembly to release the graphite capsule (piston cylinder) or furnace containing the sample capsule and support pieces (multi-anvil).
- Mount the sample in epoxy (typically to form a 25.4 mm diameter puck) (Figure 7A). Using 320 to 600 grit silicon-carbide paper, grind into the sample to expose the quenched silicate melt and metallic phases. Polish the exposed surface using either alumina or diamond suspension with decreasing grit sizes ranging from ~15 to 0.3 µm.
- Carbon coat the surface of the polished sample42 and analyze the major element composition of the metal and silicate run-products by electron probe micro analysis (EPMA). Use a defocused (10 µm) beam diameter for the silicate analysis to avoid migration of alkali elements away from the electron beam. The analytical conditions and standards used to characterize previous samples generated with the above protocol can be found in references29–31,35
Note: For experiments to investigate MSE and SSE partitioning, EPMA may also prove suitable for analysis of the tracer elements, providing they are present at sufficient concentrations. - Following major element analysis, remove the carbon coat using 0.3 µm alumina grit. Use laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) to determine the trace element content of the run-products. For an introduction to sample analysis by LA-ICPMS, please see reference43.
Note: For previous HSE solubility studies isotopes of calcium and nickel have been successfully used as internal standards to reduce the data, using both glass and sulphide reference materials respectively29,30. All analyses should be preceded by a single pass of ablation, followed by flushing the ablation cell for at least 60 sec. This ensures any surface contamination that may arise from polishing the experimental run-products does not affect the results.
Wyniki
The following examples and discussion focus on experiments to determine HSE solubility in silicate melts at low fO2. For comprehensive examples of how MSE and SSE partitioning data from multi-anvil experiments may be used to constrain the P-T-fO2 conditions of core metal segregation, the reader is referred to references9–11. Figure 7B-D displays back scattered electron images from typical experimental ru...
Dyskusje
The results of inclusion-free experiments performed using the protocols outlined here have previously been compared with literature data in references29 (Os, Ir, Au), 30 (Re, Au) and 31 (Pt). Pt is most instructive in demonstrating the usefulness of inclusion-free run-products. For experiments run at low fO2, Ertel et al.48 assigned inclusions to a stable origin and therefore restricted data reduction to the lowest counts-per-second region of time-r...
Ujawnienia
The Authors have nothing to disclose.
Podziękowania
This work was supported by the Natural Sciences and Engineering Research Council of Canada Equipment, Discovery and Discovery Accelerator Grants awarded to J.M.B. N.R.B acknowledges support from the Carnegie Institution of Washington post-doctoral fellowship program. Stephen Elardo is also thanked for his assistance prior to filming with the piston-cylinder press at the Geophysical Lab.
Materiały
| Name | Company | Catalog Number | Comments |
| G10 Epoxy/Fiberglass Sheet | Accurate plastics, Inc. | GEES.020N.3648 | |
| Powdered starting materials- -Oxides, metals, carbonates | Alfa Aesar | Specific to desired experiment | |
| Castable 2-part MgO ceramic | Aremco | Ceramcast - 584 | |
| PTFE Dry Lubricant | Camie-Campbell | 2000 TFE-Coat | |
| Graphite resistance heaters | Carbone of America (Now owned by Mersen USA) | Custom Order | |
| Barium Carbonate | Chemical Products Corporation | Custom Order | Calcined free-flowing (CFF) grade |
| C-Type Thermocouple Wire (W26%Re, W5%Re) | Concept Alloys | ~0.25 mm diameter is suitable for most experiments | |
| Zirconia Cement | Cotronics; Resbond 940 2-part cement | Use 100 parts powder for every 25 to 28 parts activator | |
| Polyvinyl Acetate (PVA) Glue | e.g., Bostik | Often sold as 'white glue' | |
| Cyanoacrylate Glue | e.g., Krazy Glue/Loctite | ||
| Piston cylinder pressure vessel and WC piston | Hi-Quality Carbide Tooling Inc. | Custom Order | |
| Silica Glass Tubing | Quartz Plus | Custom Order | |
| Crushable ZrO2 tubes | Saint-Gobain | Custom Order | |
| Crushable MgO rods and tubes | Saint-Gobain | Custom Order | |
| WC cubes for multi-anvil experiments | Tungaloy | Custom Order | Cubes are grade-F WC alloy |
| Single hole alumina tube for multi-anvil thermocouple | Vesuvius McDanel | AXS071730-04-06 | |
| 4-hole alumina tube for piston cylinder thermocouple | Vesuvius McDanel | AXF1159--07-12 | |
| 4-hole alumina tube for multi-anvil thermocouple | Vesuvius McDanel | AXF1159-04-06 |
Odniesienia
- Canup, R. M. Dynamics of Lunar Formation. Annual Review of Astronomy and Astrophysics. 42, 441-475 (2004).
- Rubie, D., Nimmo, F., Melosh, H. Formation of Earth’s core. Treatise on geophysics. 9, 51-90 (2007).
- Karato, S., Murthy, V. R. Core formation and chemical equilibrium in the Earth Physical considerations. Physics of the Earth and Planetary Interiors. 100, 61-79 (1997).
- Dziewonski, A. M., Anderson, D. L. Preliminary reference Earth model. Physics of the Earth and Planetary Interiors. 25 (4), 297-356 (1981).
- McDonough, W., Sun, S. The composition of the Earth. Chemical geology. 120 (3-4), 223-253 (1995).
- Palme, H., O’Neill, H. Cosmochemical estimates of mantle composition. Treatise on geochemistry. 2, 1-38 (2003).
- Fischer-Gödde, M., Becker, H., Wombacher, F. Rhodium, gold and other highly siderophile elements in orogenic peridotites and peridotite xenoliths. Chemical Geology. 280 (3-4), 365-383 (2011).
- Ringwood, A. E. Chemical evolution of the terrestrial planets. Geochimica et Cosmochimica Acta. 30, 41-104 (1966).
- Wood, B. J., Wade, J. Core formation and the oxidation state of the Earth. Earth and Planetary Science Letters. 236 (1-2), 78-95 (2005).
- Siebert, J., Corgne, A., Ryerson, F. Systematics of metal–silicate partitioning for many siderophile elements applied to Earth’s core formation. Geochimica et Cosmochimica Acta. 75 (6), 1451-1489 (2011).
- Righter, K. Prediction of metal–silicate partition coefficients for siderophile elements: an update and assessment of PT conditions for metal–silicate equilibrium during accretion of. Earth and Planetary Science Letters. 304 (1-2), 158-167 (2011).
- Wood, B. J., Walter, M. J., Wade, J. Accretion of the Earth and segregation of its core. Nature. 441 (7095), 825-833 (2006).
- Kimura, K. A. N., Lewis, R. O. Y. S., Anders, E. Distribution of gold and rhenium between nickel-iron and silicate melts: implications for the abundance of siderophile elements on the Earth and Moon. Geochimica et Cosmochimica Acta. 38, 683-701 (1974).
- Murthy, V. R. Early differentiation of the Earth and the problem of mantle siderophile elements: a new approach. Science. 253 (5017), 303-306 (1991).
- Righter, K., Humayun, M., Danielson, L. Partitioning of palladium at high pressures and temperatures during core formation. Nature Geoscience. 1 (5), 321-323 (2008).
- Borisov, A., Palme, H., Spettel, B. Solubility of palladium in silicate melts Implications for core formation in the Earth. Geochimica et Cosmochimica Acta. 58 (2), 705-716 (1994).
- Ertel, W., Dingwell, D. B., Sylvester, P. J. Siderophile elements in silicate melts — A review of the mechanically assisted equilibration technique and the nanonugget issue. Chemical Geology. 248 (3-4), 119-139 (2008).
- Borisov, A. L., Palme, H. The solubility of iridium in silicate melts: New data from experiments with lr10Pt90 alloys. Geochimica et Cosmochimica Acta. 59 (3), 481-485 (1995).
- Borisov, A., Palme, H. Experimental determination of Os metal/silicate partitioning. Neues Jahrbuch für Mineralogie - Abhandlungen. 172 (2-3), 347-356 (1998).
- Borisov, A., Palme, H. Experimental determination of the solubility of platinum in silicate melts. Geochimica et Cosmochimica Acta. 61 (20), 4349-4357 (1997).
- Ertel, W., O’Neill, H. S. C., Sylvester, P. J., Dingwell, D. B. Solubilities of Pt and Rh in a haplobasaltic silicate melt at 1300. C. Geochimica et Cosmochimica Acta. 63 (16), 2439-2449 (1999).
- Cottrell, E., Walker, D. Constraints on core formation from Pt partitioning in mafic silicate liquids at high temperatures. Geochimica et Cosmochimica Acta. 70 (6), 1565-1580 (2006).
- Yokoyama, T., Walker, D., Walker, R. J. Low osmium solubility in silicate at high pressures and temperatures. Earth and Planetary Science Letters. 279 (3-4), 165-173 (2009).
- Laurenz, V., Fonseca, O. C., Ballhaus, C., Peter, K., Heuser, A., Sylvester, P. J. The solubility of palladium and ruthenium in picritic melts: 2. The effect of sulfur. Geochimica et Cosmochimica Acta. 102, 172-183 (2013).
- Neill, H. S. C. Experimental petrochemisty of some highly siderophile elements at high temperatures, and some implications for core formation and the mantle’s early history. Chemical Geology. 120 (3-4), 255-273 (1995).
- Fortenfant, S. S., Dingwell, D. B., Ertel-Ingrisch, W., Capmas, F., Birck, J. L., Dalpé, C. Oxygen fugacity dependence of Os solubility in haplobasaltic melt. Geochimica et Cosmochimica Acta. 70 (3), 742-756 (2006).
- Ertel, W., O’Neill, H. S. C., Sylvester, P. J., Dingwell, D. B., Spettel, B. The solubility of rhenium in silicate melts: Implications for the geochemical properties of rhenium at high temperatures. Geochimica et Cosmochimica Acta. 65 (13), 2161-2170 (2001).
- Medard, E., Schmidt, M. W., Wahle, M., Keller, N. S., Gunther, D. Pt in Silicate Melts: Centrifuging Nanonuggets to Decipher Core Formation Processes. Lunar and Planetary Science Conference. , 3-4 (2010).
- Brenan, J., McDonough, W. Core formation and metal–silicate fractionation of osmium and iridium from gold. Nature Geoscience. 2 (11), 798-801 (2009).
- Bennett, N. R., Brenan, J. M. Controls on the solubility of rhenium in silicate melt: Implications for the osmium isotopic composition of Earth’s mantle. Earth and Planetary Science Letters. 361, 320-332 (2013).
- Bennett, N., Brenan, J., Koga, K. The solubility of platinum in silicate melt under reducing conditions: Results from experiments without metal inclusions. Geochimica et Cosmochimica Acta. 133, 422-442 (2014).
- Okamoto, H., Massalski, T. B. The Au-Pt ( Gold-Platinum ) System. Bulletin of Alloy Phase Diagrams. 6 (1), 46-55 (1985).
- Neill, H., Pownceby, M. Thermodynamic data from redox reactions at high temperatures. I. An experimental and theoretical assessment of the electrochemical method using stabilized zirconia. Contributions to Mineralogy and Petrology. 114 (3), 296-314 (1993).
- Deines, P., Nafziger, R., Ulmer, G., Woermann, E. . Temperature-oxygen fugacity tables for selected gas mixtures in the system CHO at one atmosphere total pressure. Bulletin of the Earth and Mineral Sciences Experiment Station. (88), (1974).
- Corgne, A., Keshav, S., Wood, B. J., McDonough, W. F., Fei, Y. Metal–silicate partitioning and constraints on core composition and oxygen fugacity during Earth accretion. Geochimica et Cosmochimica Acta. 72 (2), 574-589 (2008).
- Agee, C. B., Walker, D. Static compression and olivine flotation in ultrabasic silicate liquid. Journal of Geophysical Research. 93 (7), 3437-3449 (1988).
- Walker, D. Lubrication, gasketing, and precision in multianvil experiments. American Mineralogist. 76 (7-8), 1092-1100 (1991).
- Boyd, F., England, J. Apparatus for Phase-Equilibrium Measurements at Pressures up to 50 Kilobars and Temperatures up to 1750. C. Journal of Geophysical Research. 65 (2), 741-748 (1960).
- McDade, P., Wood, B. J., et al. Pressure corrections for a selection of piston-cylinder cell assemblies. Mineralogical Magazine. 66 (6), 1021-1028 (2002).
- Walker, D., Carpenter, M., Hitch, C. Some simplifications to multianvil devices for high pressure experiments. American Mineralogist. 75 (9-10), 1020-1028 (1990).
- Bertka, C. M., Fei, Y. Mineralogy of the Martian interior up to core-mantle boundary pressures. Journal of Geophysical Research. 102 (B3), 5251 (1997).
- Reed, S. J. B. . Electron Microprobe Analysis and Scanning Electron Microscopy in Geology. , (2005).
- Sylvester, P. J. . Laser Ablation ICP-MS in the Earth Sciences: Current Practices and Outstanding Issues. , (2008).
- Borisov, A., Walker, R. Os solubility in silicate melts: New efforts and results). American Mineralogist. 85 (7-8), 912-917 (2000).
- Borisov, A., Nachtweyh, K. Ru Solubility in Silicate Melts: Experimental Results in Oxidizing Region. Lunar and Planetary Science Conference. 77058, 1320 (1998).
- Borisov, A., Palme, H. Experimental determination of the solubility of Au in silicate melts. Mineralogy and Petrology. 56 (3-4), 297-312 (1996).
- Dunn, T. The Piston-Cylinder Apparatus. Short Course Handbook on Experiments at High Pressure and Applications to the Earth’s Mantle. , 39-94 (1993).
- Ertel, W., Walter, M., Drake, M., Sylvester, P. Experimental study of platinum solubility in silicate melt to 14GPa and 2273K: implications for accretion and core formation in Earth. Geochimica et Cosmochimica Acta. 70 (10), 2591-2602 (2006).
- Mann, U., Frost, D., Rubie, D. Partitioning of Ru. Rh, Pf, Re, Ir and Pt between liquid metal and silicate at high pressures and high temperatures-Implications for the origin of highly siderophile element concentrations in the Earth’s mantle. Geochimica et Cosmochimica Acta. 84, 593-613 (2012).
- Laurenz, V., Fonseca, R. O. C., Ballhaus, C., Sylvester, P. J. Solubility of palladium in picritic melts 1 . The effect of iron. Geochimica et Cosmochimica Acta. 74 (10), 2989-2998 (2010).
- Liu, Y., Ge, Y., Yu, D. Thermodynamic descriptions for Au–Fe and Na–Zn binary systems. Journal of Alloys and Compounds. 476 (1-2), 79-83 (2009).
- Rindone, G. E., Rhoads, J. L. The Colors of Platinum, Palladium, and Rhodium in Simple Glasses. Journal of the American Ceramic Society. 39 (5), 173-180 (1956).
- Akai, T., Nishii, J., Yamashita, M., Yamanaka, H. Chemical behavior of platinum-group metals in oxide glasses. Journal of Non-Crystalline Solids. 222 (Special Issue), 304-309 (1997).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone