Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Tracking Individual Running Metrics in Mice Using a Voluntary Wheel Running Protocol that Minimizes Social Isolation
W tym Artykule
Podsumowanie
This protocol describes an effective voluntary wheel-running model that can be implemented in various research contexts. Unlike other voluntary running models, this method avoids isolation housing while tracking running metrics in individual mice.
Streszczenie
Voluntary wheel running (VWR) in rodents is used to evaluate performance and endurance and to simulate exercise. In contrast to other exercise paradigms that require aversive stimulus to induce active movement, wheel running is voluntary. VWR generally consists of placing a running wheel inside the mouse home cage. However, analyzing exercise parameters can typically only be conducted when mice are individually housed, while overall cage effects can be reported when mice are group-housed. Therefore, the current models force experimenters to choose between measuring individual exercise parameters in single-housed mice or measuring group effects in group-housed mice; depending on the research question and model, this could be limiting.
Therefore, the goal of this method is to demonstrate a protocol that assesses VWR in a mouse model by quantifying running parameters in individual mice, with minimal isolation. We report on device design, preoperative preparations, and experiment setup. Results of a 6-week training protocol using this VWR model suggest it provides sufficient stimulus to increase spatial object recognition memory, comparable to the clinical effect of exercise. This simple and inexpensive model facilitates investigation into the effect of VWR on multiple measurements of wheel activity, minimizing factors that could limit or affect experimental outcomes.
Wprowadzenie
Exercise beneficially impacts physiological and psychological health and can improve the pathophysiology of numerous diseases1. Physical activity is critical for health span, and World Health Organization (WHO) reported in 2020 that increasing physical activity reduces the risk of numerous diseases, including psychological disorders2. In addition to disease prevention, exercise can enhance brain function by stimulating blood flow3, inducing neurogenesis, particularly in the hippocampus4, a key brain region for learning and memory, hormonal regulation5, and synaptic plasticity through increasing brain-derived neurotrophic factor levels6. Through these mechanisms, exercise can improve cognitive function and memory7,8,9. Additional neurobiological mechanisms promoted by exercise support mental health, including endorphin release10, cortisol reduction11, improved sleep quality12, and inhibition of neuroinflammation13. We have previously
demonstrated that voluntary exercise can promote resilience to chronic stress and anxiety-like behavior in male and female mice14. The evidence for physical activity improving outcomes in disease, combined with promoting mental health, highlight the need for strategies to encourage physical activity, including increasing our understanding of the preventative and therapeutic potential of exercise.
One method to study the effects of exercise on biological function is using rodent models of exercise. These models are effective at increasing cardiac output and inducing lactate production to various levels depending on exercise intensity and frequency. Common models used to study the therapeutic effect of exercise include swimming, treadmill training, and high-intensity interval training15. A drawback of some models, however, is involuntary or forced exercise, which could be a stressful experience. For example, when mice are subjected to treadmill running in a model cerebral ischemia injury, elevated anxiety-like behavior in the open field test, increased corticosterone levels, neuronal damage or increased cytokine levels have been reported16. Alternatively, voluntary exercise models, including VWR, can avoid the potentially stressful experience, allowing rodents to freely run, without the need for conditioning or reinforcement17. VWR is typically performed by providing continuous access to a running wheel in a cage housing 4-5 mice or cages of single-housed mice. Therefore, VWR provides non-stressful conditions, as it does not require a negative stimulus or a direct intervention from the experimenter and may provide a coping mechanism or enrichment experience18,19,20.
The goal of this method is to propose a simple and inexpensive VWR protocol that enables the tracking of individual running metrics with minimal isolation. Mice are group-housed to avoid prolonged social isolation, and running parameters are recorded for individual mice by isolating mice in a running arena for only 2 h/day. Details are provided for a 6 week VWR model in which 10 C57BL/6 mice had access to running wheels for 2 h/day while 10 C57BL/6 mice had access to locked wheels. Results confirmed that this VWR protocol was successfully able to track individual running parameters with minimal isolation and mediate the beneficial effects of exercise on hippocampal-induced spatial memory in the spatial object recognition test (SORT).
Protokół
All experimental procedures were approved by Rowan University's Animal Care and Use Committee. Male mice weighed between 20 g and 27 g and were 8-10 weeks old at the start of the study. Mice were housed in groups of 4, in a controlled environment (12:12 h light/dark cycle) with ad libitum access to food and water.
1. Wheels setup
- On the outer middle circumference of each wheel, glue one magnet. This magnet will be detected by the censor of the speedometer to monitor VWR (Figure 1A).
- On the opposite side of the wheel, glue an object of similar weight to the magnet (a coin) to counterbalance and prevent unwanted rotation due to the weight of the magnet.
- Create a stage for the speedometer and wheel. Using a white foam board, cut a piece of complementary shape to the base of the wheel and tape it to the base (Figure 1B).
- After inserting a fresh set of batteries into the speedometer, set the wheel size during initial programming by measuring the outer circumference of the wheel.
- Adhere the speedometer censor to the platform. Ensure the censor of the speedometer is placed parallel to the magnet of the wheel to allow for proper recording of movement (Figure 1C). Each time the magnet passes the sensor, it will count as one revolution.
- Wrap the censor and wire with duct tape to avoid wire chewing by mice (Figure 1D).
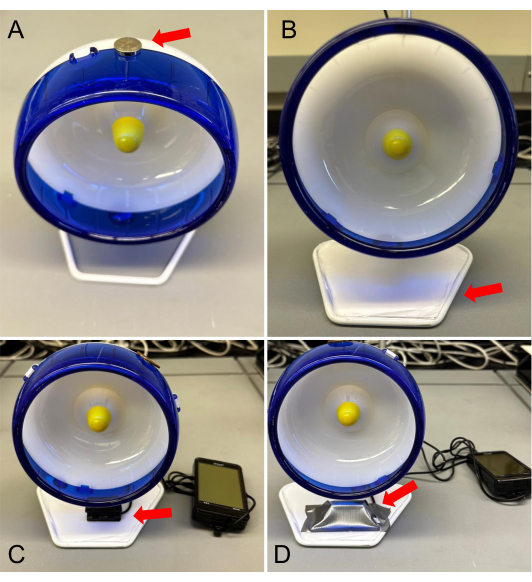
Figure 1: Running wheel apparatus setup. (A) A magnet and a coin glued (red arrow) on opposite sides of the outer edge of the wheel. (B) A platform made of foam board (red arrow) used as a stage for the speedometer' censor. (C) Speedometer's censor on platform (red arrow); (D) Taped censor (red arrow) and finalized wheel setup. Please click here to view a larger version of this figure.
2. Apparatus setup
- Utilize the behavioral apparatus and divide the 40 cm x 40 cm x 35 cm (h) box into four quadrants to individually test four mice at the same time.
- Cut two white foam board pieces based on the following dimensions: L 30 cm, W 40 cm (Figure 2A).
- In the middle of the 40 cm wide side of the foam board, make a 20 cm cut to enable the intersection of two foam boards.
- Cover the foam board with waterproof adhesive paper to allow for easy cleaning between groups.
- Divide the 40 cm x 40 cm x 35 cm (h) box into four quadrants (20 cm x 20 cm x 30 cm (h) using two intersecting foam boards (Figure 2B).
- Place one prepared wheel (check wheel setup above) in the corner of every quadrant and tape wire to the wall. Tape speedometers to the side of the clear Perspex apparatus (Figure 2C).
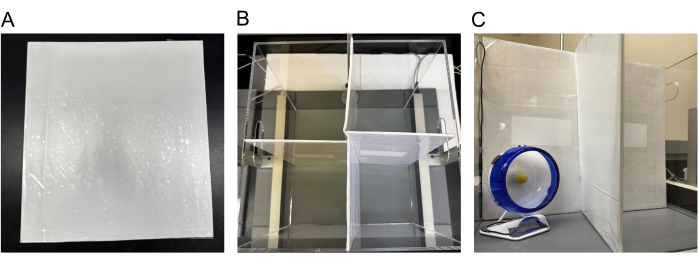
Figure 2: Apparatus setup. (A) Foam board cut and taped; (B) Perspex apparatus divided into four quadrants; (C) one wheel placed in the corner of a quadrant with speedometer wire taped on the wall. Please click here to view a larger version of this figure.
3. VWR
NOTE: In addition to speedometer metrics, we utilized behavioral tracking software, the ANY-maze behavioral tracking system, to provide additional behavioral tracking information.
- Carry out the VWR sessions daily, in the light-phase at the same time each day (from 9 a.m. to 11:00 a.m). Place the no-exercise mice in identical arenas for 2 h but with locked wheels. Lock the wheels using hot glue to immobilize the wheel's rotation center and disable rotation.
- Place the mice in the VWR room to habituate for 30-60 min.
- Reset all speedometers.
- Place each mouse in the corner of the quadrant where it can freely run for 2 h.
- After 2 h, return the mice back to their home cage and record the speedometer data.
- Clean the arenas and wheels with 70% ethanol between both groups.
4. SORT
NOTE: At the end of 5 weeks of VWR, SORT was conducted to test whether exercise improved hippocampal function according to the published protocol21.
- Carry out the habituation sessions and SORT during the light phase in a room with ambient lighting. Conduct habituation sessions a day prior to SORT during which move the mice into individual cages and bring them into the testing room for at least 30 min of acclimation.
- Assign one of the corners of the arena as the "release corner" and make sure that every mouse is placed facing the release corner to freely explore the arena for 6 min. Return the mice to their individual cage to clean the arena with 70% ethanol.
- Repeat step 4.1.1 2x so that each mouse goes through three habituation sessions. Once the habituation sessions are completed, return all mice to their home cages.
- After 24 h, return the mice to the testing room and allow them to habituate in individual cages for 30-60 min prior to testing.
- Affix two different objects in a counterbalanced way in the arena 6 x 6 cm away from two non-release corners.
- Place each mouse facing the release corner to freely explore the objects for 10 min. Return the mice back to their individual cages for 20 min and clean the objects and arenas with 70% ethanol.
- Move one of the two objects and affix it 6 x 6 cm away from a new non-release corner without modifying the location of the other object.
- Place each mouse facing the release corner to freely explore the objects for 10 min before going back to their home cage. Record this test using automated software to measure the investigation time per object and calculate the percent investigation time as follows:
% investigation time =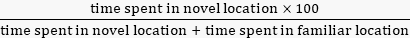
5. Statistical analysis
- Express all data as mean ± SEM.
- Perform statistical analyses using statistical analysis software with significance set at p ≤ 0.05. Analyze the SORT results using the Student's T-test.
Wyniki
In this study, 20 C57BL/6 male mice were randomly assigned to one of the two groups: no-exercise (NO-EX) or exercise (VWR) for 6 weeks. Individual running parameters were recorded daily on speedometers and plotted below ± SEM (Figure 3A-C). Throughout the 6 weeks, mice were most active in week 2, during which they spent on average 2,135.87 ± 351.14 s on the wheel, traveling a distance of 1.16 ± 0.25 km at a speed of 2.25 ± 0.14 km/h. In contrast, the lowe...
Dyskusje
Various exercise paradigms are used by researchers to evaluate physical activity and its effects in rodents, including voluntary and forced running paradigms. Compared to the forced exercise paradigms, VWR allows mice to freely run at a lower intensity, which minimizes stressful conditions22. Currently, investigators can collect exercise parameters of individual mice by single-housing them23,24,25,
Ujawnienia
The authors have no conflicts of interest to disclose.
Podziękowania
None
Materiały
| Name | Company | Catalog Number | Comments |
| ANY-box 5-Behavior Test System | Stoelting | 65000 | https://stoeltingco.com/Neuroscience/ANY-box-Multi-Configuration-Behavior-Apparatus~9838 |
| Bike Computer Bicycle Wired Speedometers and Odometers | IPSXP | N/A | Any wired speedometer that meausre distsnce, time, speed could work |
| Clear Adhesive Protective Liner | Con-Tact Brand | N/A | https://con-tactbrand.com/products/clear-cover%E2%84%A2-clear-matte?_pos=1&_fid=be2dc000c&_ss=c |
| GraphPad Prism | |||
| Instant Adhesive 496 Super Bonder | Loctite | S-24546 | https://www.uline.com/Product/Detail/S-24546/Adhesives-Glue-Epoxy/Loctite-Instant-Adhesive-496-Super-Bonder?pricode=WB0948&gadtype=pla&id= S-24546&gad_source=1&gclid=Cjw KCAjw68K4BhAuEiwAylp3klS0FN Ur-l_xKkue1NUmruDDV48QO5b2tVH W9Bc08s9eZKmedj-yxBoCgIEQAvD_BwE |
| Pro Mag Neodymium Magnet | Applied Magnets | ND018-6 | If choosing other magnets, make sure they are strong enough to be detected by the censor |
| Silent Spinner Small Animal Exercise Wheels (mini 11.4 cm) | Kaytee | SKU# 100079369 | https://www.kaytee.com/all-products/ small-animal/silent-spinner-wheel |
| White Foam Boards | Pacon | 5553 | https://pacon.com/foam-board-350/pacon-foam-board-20-x-30-white-10pkg-2.html |
Odniesienia
- Bull, F. C., et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 54 (24), 1451-1462 (2020).
- . Physical activity Available from: https://www.who.int/health-topics/physical-activity (2024)
- Gliemann, L., Vestergaard Hansen, C., Rytter, N., Hellsten, Y. Regulation of skeletal muscle blood flow during exercise. Curr Opin Physiol. 10, 146-155 (2019).
- Liu, P. Z., Nusslock, R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. 12, 52 (2018).
- Hackney, A. C., Lane, A. R. Exercise and the regulation of endocrine hormones. Prog Mol Biol Transl Sci. 135, 293-311 (2015).
- De la Rosa, A., et al. Long-term exercise training improves memory in middle-aged men and modulates peripheral levels of BDNF and Cathepsin B. Sci Rep. 9 (1), 3337 (2019).
- De Miguel, Z., et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature. 600 (7889), 494-499 (2021).
- Roig, M., Nordbrandt, S., Geertsen, S. S., Nielsen, J. B. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci Biobehav Rev. 37 (8), 1645-1666 (2013).
- Babaei, P., Azari, H. B. Exercise training improves memory performance in older adults: A narrative review of evidence and possible mechanisms. Front Hum Neurosci. 15, 771553 (2022).
- Harber, V. J., Sutton, J. R. Endorphins and exercise. Sports Med. 1 (2), 154-171 (1984).
- De Nys, L., et al. The effects of physical activity on cortisol and sleep: A systematic review and meta-analysis. Psychoneuroendocrinology. 143, 105843 (2022).
- Alnawwar, M. A., et al. The effect of physical activity on sleep quality and sleep disorder: A systematic review. Cureus. 15 (8), e43595 (2023).
- Mee-inta, O., Zhao, Z. -. W., Kuo, Y. -. M. Physical exercise inhibits inflammation and microglial activation. Cells. 8 (7), 691 (2019).
- Elias, E., Zhang, A. Y., White, A. G., Pyle, M. J., Manners, M. T. Voluntary wheel running promotes resilience to the behavioral effects of unpredictable chronic mild stress in male and female mice. Stress. 26 (1), 2203769 (2023).
- Hastings, M. H., et al. Animal models of exercise from rodents to pythons. Circ Res. 130 (12), 1994-2014 (2022).
- Svensson, M., et al. Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiol Stress. 5, 8-18 (2016).
- Manzanares, G., Brito-da-Silva, G., Gandra, P. G. Voluntary wheel running: patterns and physiological effects in mice. Braz J Med Biol Res. 52 (1), e7830 (2018).
- Garland, T., et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 214 (Pt 2), 206-229 (2011).
- Meijer, J. H., Robbers, Y. Wheel running in the wild. Proc Biol Sci. 281 (1786), 20140210 (2014).
- Banjanin, S., Mrosovsky, N. Preferences of mice, Mus musculus, for different types of running wheel. Lab Anim. 34 (3), 313-318 (2000).
- Denninger, J. K., Smith, B. M., Kirby, E. D. Novel object recognition and object location behavioral testing in mice on a budget. J Vis Exp. (141), e58593 (2018).
- Manzanares, G., Brito-da-Silva, G., Gandra, P. G. Voluntary wheel running: patterns and physiological effects in mice. Braz J Med Biol Res. 52 (1), e7830 (2018).
- Scott, K. S., Chelette, B., Chidomere, C., Phillip West, A., Dantzer, R. Cisplatin decreases voluntary wheel-running activity but does not impair food-motivated behavior in mice. Brain Behav Immun. 111, 169-176 (2023).
- Hamm, S. E., et al. Prolonged voluntary wheel running reveals unique adaptations in mdx mice treated with microdystrophin constructs ± the nNOS-binding site. Front Physiol. 14, 1166206 (2023).
- Williams, Z. A. P., et al. Sex-specific effects of voluntary wheel running on behavior and the gut microbiota-immune-brain axis in mice. Brain Behav Immun Health. 30, 100628 (2023).
- Ehichioya, D. E., et al. Gut microbiota depletion minimally affects the daily voluntary wheel running activity and food anticipatory activity in female and male C57BL/6J mice. Front Physiol. 14, 1299474 (2023).
- Kim, M. -. J., et al. Sex differences in body composition, voluntary wheel running activity, balance performance, and auditory function in CBA/CaJ mice across the lifespan. Hear Res. 428, 108684 (2023).
- Yusifova, M., et al. Voluntary wheel running exercise does not attenuate circadian and cardiac dysfunction caused by conditional deletion of Bmal1. J Biol Rhythms. 38 (3), 290-304 (2023).
- Bittel, A. J., Bittel, D. C., Gordish-Dressman, H., Chen, Y. -. W. Voluntary wheel running improves molecular and functional deficits in a murine model of facioscapulohumeral muscular dystrophy. iScience. 27 (1), 108632 (2024).
- Leimbacher, A. C., et al. Voluntary exercise does not always suppress lung cancer progression. iScience. 26 (8), 107298 (2023).
- Zhang, Y., et al. Trimethylamine N-oxide aggravated cognitive impairment from APP/PS1 mice and protective roles of voluntary exercise. Neurochem Int. 162, 105459 (2023).
- Zhang, W., et al. Notch1 is involved in physiologic cardiac hypertrophy of mice via the p38 signaling pathway after voluntary running. Int J Mol Sci. 24 (4), 3212 (2023).
- Wan, C., et al. Long-term voluntary running improves cognitive ability in developing mice by modulating the cholinergic system, antioxidant ability, and BDNF/PI3K/Akt/CREB pathway. Neurosci Lett. 836, 137872 (2024).
- Weegh, N., et al. Wheel running behaviour in group-housed female mice indicates disturbed wellbeing due to DSS colitis. Lab Anim. 54 (1), 63-72 (2020).
- Coleman, A. L., et al. Short-term social isolation induces depressive-like behaviour and reinstates the retrieval of an aversive task: Mood-congruent memory in male mice. J Psychiatry Neurosci. 38 (4), 259-268 (2013).
- Desan, P. H., Silbert, L. H., Maier, S. F. Long-term effects of inescapable stress on daily running activity and antagonism by desipramine. Pharmacol Biochem Behav. 30 (1), 21-29 (1988).
- DeVallance, E., et al. Effect of chronic stress on running wheel activity in mice. PloS One. 12 (9), e0184829 (2017).
- Liu, P. Z., Nusslock, R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. 12, 52 (2018).
- Erickson, K. I., et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 108 (7), 3017-3022 (2011).
- Zhang, S., et al. The effect of aerobic exercise on cognitive function in people with Alzheimer's disease: A systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health. 19 (23), 15700 (2022).
- Nystoriak, M. A., Bhatnagar, A. Cardiovascular effects and benefits of exercise. Front Cardiovasc Med. 5, 135 (2018).
- Kirwan, J. P., Sacks, J. P., Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 84 (7 Suppl 1), S15-S21 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone