Для просмотра этого контента требуется подписка на Jove Войдите в систему или начните бесплатную пробную версию.
A CRISPR-Cas9 Technique for Gene Editing in T Cells
Overview
This video demonstrates an assay for performing gene editing in human T cells using the CRISPR-Cas9 technology. A mixture of primary CD4+ and CD8+ T cells is combined with a CRISPR-Cas9 ribonucleoprotein complex, targeting specific genes for knockout. Upon electroporation, the sgRNA guides Cas9 to the target DNA sequence, creating precise cuts. These cuts are then repaired by the cell's non-homologous end-joining mechanism, leading to gene knockout.
протокол
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Designing of sgRNAs and gene disruption in primary human T cells
- Designing CRISPR sgRNA's
NOTE: Several servers and programs facilitate the design of target-specific single guide RNAs (sgRNA). In this protocol, clustered regularly interspaced short palindromic repeats (CRISPR) sgRNAs were designed using CHOPCHOP (https://chopchop.cbu.uib.no), and the gRNA design portal from the Broad Institute (https://portals.broadinstitute.org/gpp/public/analysis-tools/sgrna-design).- For each target gene, design six to ten sgRNA sequences to target early coding exon sequences.
NOTE: sgRNA should have high on-target efficacy and low off-target efficacy. Each machine learning algorithm for determining sgRNA efficacy works slightly differently. Therefore, comparing multiple sgRNA design tools and curating a list of six to ten sgRNA for screening is recommended.
- For each target gene, design six to ten sgRNA sequences to target early coding exon sequences.
- Gene disruption in primary human T-cells
- Obtain autologous peripheral blood mononuclear cells (PBMC's) from healthy volunteer donors. A schematic timeline of the protocol is described in Figure 1A and described below.
- Isolate CD4+ and CD8+ T-cells using commercially available CD4 and CD8 selection kits.
- Combine CD4+ and CD8+ T-cells at a 1:1 ratio and incubate overnight at 3x106 cells/mL in R10 supplemented with 5 ng/mL human interleukin-7 (huIL-7) and human interleukin -15 (huIL-15) each. Addition of IL-7 and IL-15 is recommended as they are known to promote a central memory phenotype and chimeric antigen receptor (CAR)-T cells expanded with IL-7 and IL-15 have superior anti-tumor efficacy compared to interleukin-2 (IL-2) expanded CAR-T cells.
- The next day, count T-cells and centrifuge 5-10 x 106 cells at 300 x g for 5 min. Discard all the supernatant and wash the cell pellet in reduced-serum minimal essential media. Resuspend the pellet in 100 µL of nucleofection solution according to the manufacturer's instructions (Table of Materials).
- While washing the cells, prepare ribonucleoprotein (RNP) complex with CRISPR-associated protein 9 (Cas9) and gRNA by incubating 10 µg of Cas9 nuclease with 5 µg of sgRNA for 10 min at room temperature (RT). The molar ratio of Cas9 to sgRNA is 1:2.4. A mock control that contains Cas9 and an electroporation enhancer, but no sgRNA, is recommended.
NOTE: Multiplex gene editing can be performed at this step by making RNP complexes for each target separately and combining them with cells at the time of nucleofection, as described in the next step. Choosing the reagents to assemble the RNP complex is key to getting high knockout (KO) efficiency. For example, Cas9 nucleases from different vendors have varying off-target effects, and chemically modified gRNA decreases toxicity to T cells, thereby increasing KO efficiency. - Combine the resuspended cells from step 1.2.4 with the RNP complex from step 1.2.5 and add 4.2 µL of 4 µM electroporation enhancer (single-stranded DNA [ssDNA] oligonucleotide that is non-homologous to the human genome). Mix well and transfer into electroporation cuvettes. Avoid bubbles as they impair electroporation efficiency.
- Electroporate cells using pulse code EH111. After electroporation, incubate cells in R10 supplemented with 5 ng/mL huIL-7 and huIL-15 at 5x106 cells/mL at 30 °C for 48 h in 12-well plates. After 48 h, proceed with T-cell activation and expansion.
Результаты
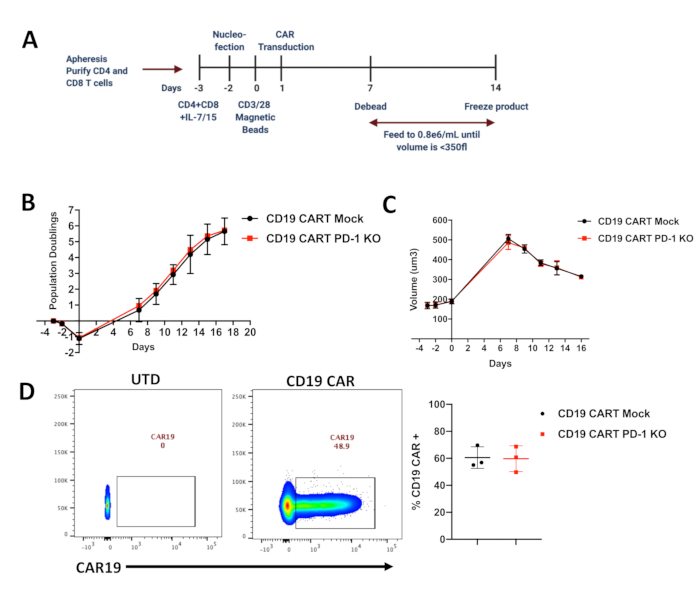
Figure 1. Expansion of edited CAR-T cells and their population doublings. (A) Timeline of CRISPR editing and manufacturing in primary human CART cells. (B) Population Doublings in Mock and CRISPR-edited CD19 CAR-T cells measured using a Coulter Counter during the expansion of the CAR-T cells (n=3 healthy donors; KO=knockout) (C) ...
Раскрытие информации
Материалы
| Name | Company | Catalog Number | Comments |
| 4D-Nucleofactor Core Unit | Lonza | AAF-1002B | |
| 4D-Nucleofactor X-Unit | Lonza | AAF-1002X | |
| Cas9-Electroporation enhancers | IDT | 1075915 | |
| CD4+ T cell isolation Kit | StemCell technologies | 15062 | |
| CD8+ T cell isolation Kit | StemCell technologies | 15063 | |
| Corning 0.45 micron vacuum filter/bottle | Corning | 430768 | |
| Corning T150 cell culture flask | Millipore Sigma | CLS430825 | |
| DNAeasy Blood and Tissue Kit | Qiagen | 69504 | |
| Glutamax supplement | ThermoFisher | 35050061 | |
| HEPES (1 M) | ThermoFisher | 15630080 | |
| huIL-15 | PeproTech | 200-15 | |
| huIL-7 | PeproTech | 200-07 | |
| Lipofectamine 2000 | ThermoFisher | 11668019 | |
| Opti-MEM | ThermoFisher | 31985062 | |
| P3 Primary cell 4D-nucleofactor X Kit L | Lonza | V4XP-3024 | |
| Penicilin-Streptomycin-Glutamine | ThermoFisher | 10378016 | |
| RPMI1640 | ThermoFisher | 12633012 | |
| sgRNA | IDT | ||
| Spy Fi Cas9 | Aldevron | 9214 |
This article has been published
Video Coming Soon
Source: Agarwal, S., et al. Production of Human CRISPR-Engineered CAR-T Cells. J. Vis. Exp. (2021).
Авторские права © 2025 MyJoVE Corporation. Все права защищены