Для просмотра этого контента требуется подписка на Jove Войдите в систему или начните бесплатную пробную версию.
Method Article
Абляция глазных стебельков для увеличения созревания яичников у грязевых крабов
В этой статье
Erratum Notice
Резюме
Два протокола абляции глазных стебельков (т.е. прижигание и хирургическое вмешательство) были выполнены на анестезированных самках крабов. Абляция глазного стебля грязевых крабов ускорила созревание яичников без снижения выживаемости.
Аннотация
Грязевые крабы (Scylla spp.) являются коммерчески важными видами ракообразных, которые можно найти по всему Индо-Западному Тихоокеанскому региону. Во время культивирования индукция созревания яичников важна для удовлетворения потребительского спроса на зрелых грязевых крабов и ускорения производства семян. Абляция глазного стебля является эффективным инструментом для ускорения созревания яичников у грязевых крабов. Тем не менее, не существует стандартного протокола для абляции глазного стебля грязевых крабов. В этом исследовании описаны два метода абляции глазных стебельков: прижигание (использование горячего металла для удаления глазного стебля анестезированного краба) и хирургическое вмешательство (удаление глазного стебля с помощью хирургических ножниц). Перед абляцией глазного стебля половозрелые самки (CW > 86 мм) были анестезированы с помощью пакета со льдом (-20 ° C) с морской водой. Когда температура воды достигала 4 °C, мешок со льдом вынимали из воды. Проточная морская вода (температура окружающей среды: 28 °C) использовалась для восстановления после анестезии сразу после абляции глазного стебля. Смертность не наступала ни во время, ни после процесса абляции глазных стебельков. Представленный здесь протокол абляции глазных стебельков ускорил созревание яичников грязевых крабов.
Введение
Все четыре вида грязевых крабов, принадлежащие к роду Scylla, являются коммерчески важными видами ракообразных в аквакультуре 1,2. Рост ракообразных, в том числе грязевых крабов, и их трансформация из преждевременной (субвзрослой или пубертатной) фазы в половозрелую (взрослую) фазу происходят в процессе линьки, который включает периодическое сбрасывание старых и меньших экзоскелетов. Ширина панциря (CW), хелипеды и морфология брюшного лоскута широко используются для определения половой зрелости Scylla spp. 3,4,5. Процесс линьки регулируется действием различных гормонов и требует огромного количестваэнергии6. В дополнение к нормальному процессу линьки, потеря конечностей, добровольно или вызванная внешними факторами, ускоряет линьку крабов, не влияя на их выживаемость 7,8,9. Поэтому аутотомия конечностей обычно используется для индукции линьки в индустрии разведения грязевых крабовс мягким панцирем 7,9.
Односторонняя или двусторонняя абляция глазного стебля в основном популярна у пресноводных креветок и морских креветок для созревания гонад и производства семян 10,11,12,13. Распространенные методы абляции глазного стебля у ракообразных включают следующее: (i) лигирование у основания глазного стебля с использованием нити14,15; (ii) прижигание глазного стебля с помощью горячих щипцов или электрокоагуляционных устройств16; (iii) удаление или прямое защемление глазного стебля, чтобы оставить открытую рану12; и (iv) удаление содержимого глазного стебля через разрез после разрезания дистальной части глаза бритвой17. Х-органы глазного стебля являются важными эндокринными органами у ракообразных, поскольку они регулируют гипергликемические гормоны ракообразных (CHH), гормоны, ингибирующие линьку (MIH), и гормоны, ингибирующие вителлогенез (VIH)6,18,19,20,21,22. Х-органы глазного стебля (или комплекс синусовых желез) синтезируют и высвобождают гормоны, ингибирующие гонады (GIH), также известные как гормоны, ингибирующие вителлогенез (VIH), принадлежащие к семейству нейропептидных гормонов6. Односторонняя или двусторонняя абляция глазных стебельков снижает синтез GIH, что приводит к доминированию стимулирующих гормонов (т.е. гонадстимулирующих гормонов, GSH) и ускорению процесса созревания яичников у ракообразных23,24,25,26. Без влияния ГИГ после абляции глазных стебельков самки ракообразных посвящают свою энергию развитию яичников27. Было обнаружено, что односторонняя абляция глазных стебельков достаточна для индукции созревания яичников у ракообразных11 и что абляция глазного стебля креветок и крабов может восстанавливаться после нескольких линьк28. У Scylla spp. зарегистрировано четыре стадии развития яичников: i) незрелая (стадия-1), ii) раннеспелая (стадия-2), iii) предзрелая (стадия-3) и iv) полностью зрелая (стадия-4)29,30. Незрелая стадия яичников встречается у неполовозрелых самок. После пубертатной линьки и спаривания незрелая завязь начинает развиваться и, наконец, созревает (стадия-4) до нереста31.
Протокол абляции глазного стебля необходим для развития маточного стада грязевых крабов и производства семян. На мировом продовольственном рынке потребители предпочитают зрелых грязевых крабов с полностью зрелыми яичниками (стадия 4), а не крабов с более высоким содержанием мышц и, таким образом, имеют более высокую коммерческую ценность, даже выше, чем крупные самцы. Не существует полного протокола абляции глазного стебля грязевых крабов. Протокол абляции глазных стебельков в этой работе сводит к минимуму стресс за счет использования полностью анестезированных крабов и сводит к минимуму физические травмы персонала от укусов крабов. Этот протокол прост и экономичен. Здесь мы представляем протокол абляции глазных стебельков Scylla spp., который может вызвать созревание гонады. Были протестированы два метода абляции глазных стекол (прижигание и хирургическое вмешательство), и их эффективность сравнивалась на основе скорости развития гонад самок грязевых крабов.
протокол
Этот протокол соответствует Малайзийскому кодексу практики по уходу за животными и их использованию в научных целях, изложенным Ассоциацией лабораторных животных Малайзии. Принесение в жертву экспериментальных образцов было сделано в соответствии с Руководством Национального института здравоохранения по уходу и использованию лабораторных животных (NIH Publications No 8023, пересмотрено в 1978 году). Половозрелые самки грязевых крабов (оранжевый грязевой краб S. olivacea) были собраны на местном рынке (5°66′62′′ с.ш., 102°72′33′′ в.д.) на водно-болотных угодьях Сетиу в Малайзии. Вид грязевого краба был идентифицирован по морфологическим признакам1.
1. Отбор проб и дезинфекция
- Собирайте здоровых, активных и недоношенных самок грязевых крабов (рис. 1).
ПРИМЕЧАНИЕ: Недоношенные самки крабов имеют треугольные и светлые брюшные лоскуты вместе с диапазоном CW 80−85 мм. - Промойте крабов хлорированной водопроводной водой (пресной), чтобы удалить мусор и осмофильных паразитов.
- Замочите крабов в формальдегиде 150 ppm с соленостью 20 ppt на 30 минут.
- Поддерживайте непрерывную и бережную аэрацию воздушными камнями во время обработки формальдегидом. Источником аэрации может быть либо центральная линия аэрации, либо насос аквариумного аэратора.
- Промойте крабов проточной морской водой, чтобы удалить остатки формальдегида.
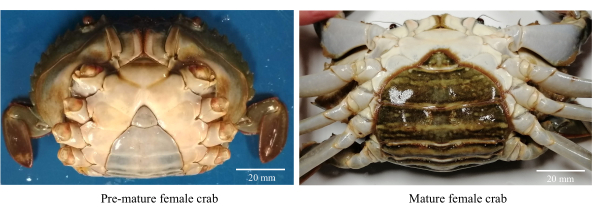
Рисунок 1: Морфология брюшной полости самок грязевых крабов, используемая для определения стадий полового созревания. Пожалуйста, нажмите здесь, чтобы просмотреть увеличенную версию этого рисунка.
2. Акклиматизация
- Перенесите каждую продезинфицированную самку в отдельный круглый резервуар объемом 32 л.
- Выращивайте самок в течение 3 дней при солености 20 ppt и продолжайте кормить два раза в день (утром в 09:00 и вечером в 20:00) нарезанной морской рыбой в количестве около 4-5% от массы тела краба.
- Убирают лишние и несъеденные корма сифоном перед утренним кормлением.
- Ежедневно обменивайте 10% морской воды для выращивания крабов (20 ppt).
3. Индуцированная линька для половой зрелости
- Отрежьте все ноги, кроме плавательных, стерилизованными ножницами.
- Поймайте краба сачком и осторожно держите краба. Сначала обрежьте ножницами обе хелипеды, а затем ходовые ноги на втором суставе. Краб автоматически автотомирует поврежденные придатки. Анестезия не требуется при аутотомии конечности.
- Вымойте краба в пресной воде сразу после аутотомии конечности.
- По отдельности переместите автотомированных крабов в перфорированные пластиковые корзины (28 см Д x 22 см Ш x 7 см В) и поместите их в резервуар из стекловолокна (305 см Д x 120 см Ш x 60 см В).
ПРИМЕЧАНИЕ: Две корзины можно связать и скрепить вместе. Верхняя корзина используется в качестве крышки, чтобы краб не смог вырваться из корзины. - Используйте рециркуляционную систему аквакультуры (RAS) с соленостью 20 ppt и глубиной воды не менее 10 см, чтобы убедиться, что вся пластиковая корзина погружена в воду.
- Продолжайте кормить самку краба с автотомией двух раз в день нарезанной морской рыбой на 5-7% от массы тела краба.
- Выращивайте крабов до полового созревания путем линьки (35 дней).
ПРИМЕЧАНИЕ: Индуцированная линька может быть пропущена для коммерческого созревания яичников и производства семян с дикими зрелыми самками грязевых крабов. Добытые половозрелые самки из дикой природы должны быть акклиматизированы и непосредственно подвергнуты анестезии холодовым шоком и последующей абляции глазных стебельков.
4. Анестезия
- Отбирают половозрелых самок с брюшным лоскутом овальной формы темного цвета с CW >86 мм (рис. 1).
- Ловите крабов сачком, и держите их по отдельности в небольших аквариумах для анестезии.
- После 5 минут акклиматизационного периода добавьте 2-феноксиэтанол (2-PE) в количестве 2 мл / л в каждый аквариум и дайте 15 минут анестезии.
- Убедитесь, что крабы полностью обезболены из-за отсутствия спонтанного движения.
5. Абляция глазных стебельков
- Техника прижигания
- Выполняйте все процедуры поверх стола и на открытой площадке.
- Возьмите металлический стержень из никелевой стали с плоской головкой (например, отвертку) с деревянной или пластиковой ручкой и накройте ручку влажным хлопчатобумажным полотенцем.
- Стерилизуйте два хирургических щипца из нержавеющей стали в автоклаве.
- Приготовьте 70% этанол в пульверизаторе и держите его подальше от любых источников, связанных с огнем, таких как паяльная лампа и раскаленная отвертка. Подготовьте папиросную бумагу к использованию.
ПРИМЕЧАНИЕ: Этанол легко воспламеняется. Держитесь на безопасном расстоянии от источников огня. - Надежно подсоедините паяльную лампу к газовому баллону (бутану).
ВНИМАНИЕ: Следуйте инструкциям на паяльной лампе и газовом баллоне. Убедитесь, что паяльная лампа выключена при соединении с газовым баллоном. Прочтите и соблюдайте все меры пожарной безопасности, указанные на газовом баллоне. - Надевайте плотные хлопчатобумажные перчатки, чтобы избежать травм от горячих предметов.
- Подвергните кончик металлического стержня огню паяльной лампы, пока металлический стержень не станет ярко-красным.
- Накройте обезболивающего краба влажным хлопчатобумажным полотенцем.
ПРИМЕЧАНИЕ: Накройте усики краба, чтобы избежать ненужных повреждений. - Зажмите один глаз краба стерилизованными щипцами.
ПРИМЕЧАНИЕ: Стерилизуйте щипцы в автоклаве для первого использования и продезинфицируйте с использованием 70% этанола для последующего использования на других крабах. - Держите раскаленный металлический плоский наконечник на глазу краба и слегка надавливайте примерно на 10−15 с, пока стебель глаза не приобретет оранжевый или красновато-оранжевый цвет. Будьте осторожны при проведении этого этапа, чтобы не повредить соседние конструкции.
ПРИМЕЧАНИЕ: Для проведения абляции глазных стебельков после метода прижигания необходимы два человека: один для удержания краба, а другой для выполнения процедуры абляции. - Продезинфицируйте щипцы 70% этаноловым спреем, чтобы избежать перекрестного загрязнения между крабами.
ПРИМЕЧАНИЕ: Выполняйте этот шаг только в течение 5 минут после процедуры абляции глазных стебельков, чтобы убедиться, что щипцы остыли перед дезинфекцией с использованием 70% этанола для предотвращения потенциальной опасности возгорания. - После проведения абляции глазного стебля у всех крабов окуните горячий металлический стержень из никелевой стали (отвертку) в водопроводную воду.
- Продезинфицируйте полотенце перед повторным использованием. Для экономии времени можно использовать несколько полотенец.
ПРИМЕЧАНИЕ: Вымойте полотенце водопроводной водой и опустите его в хлорированную воду с концентрацией 30 ppm на 5 минут. Затем снова промойте полотенце водопроводной водой и опустите его в раствор тиосульфата натрия 1 г/л. - Держите паяльную лампу в надежном месте после выключения и подождите, пока она не нагреется до температуры окружающей среды (около 30 минут), прежде чем отключать.
- Техника операции
- Выполняйте процедуру в хорошо проветриваемом помещении.
- Стерилизуйте два хирургических ножницы и щипцы в автоклаве.
- Налейте 50 мл 70% этанола в стеклянный стакан объемом 100 мл.
- Наденьте плотные хлопчатобумажные перчатки.
- Возьмите обезболивающего краба и накройте его влажным хлопчатобумажным полотенцем.
- Зажмите один глаз краба стерилизованными щипцами.
- Быстро отрежьте глазной стебель с помощью стерилизованных хирургических ножниц.
ПРИМЕЧАНИЕ: Гемолимфа может быть потеряна из раненой части краба. - Окуните ножницы и щипцы в 70% этанола после каждого использования и высушите их папиросной бумагой перед повторным использованием.
6. Постанестезиологический уход
- Приготовьте 20 ppt фильтрованной морской воды и храните в верхнем резервуаре с непрерывной аэрацией.
- Соедините гибкую трубу с верхним баком для гравитационного потока воды.
- Сразу после абляции глазного стебля поместите краба в корзину и подвергните краба проточной морской воде (температура окружающей воды: 28 ° C) из верхнего резервуара.
- Следите за потоком морской воды и следите за крабом до тех пор, пока он не сможет двигаться самопроизвольно, что указывает на восстановление после анестезии.
ПРИМЕЧАНИЕ: Морская вода может быть приготовлена в наземном резервуаре, а для потока воды можно использовать погружной водяной насос. - Держите крабов по отдельности в морской воде с аэрацией 20 ppt в аквариуме в течение 30 минут для дальнейшего наблюдения.
ПРИМЕЧАНИЕ: Извлеченные крабы будут культивироваться индивидуально в последующем процессе выращивания маточного стада.
7. Наблюдение за созреванием яичников
- Выращивание маточного стада
- Переложите зрелых крабов в отдельные круглые резервуары объемом 32 л.
- Продолжайте кормить нарезанной морской рыбой (замороженной при -20 ° C) два раза в день (утром в 09:00 и вечером в 20:00) и удаляйте несъеденный корм перед утренним кормлением.
- Выращивайте маточное стадо индивидуально в течение 30 дней при солености 20 ppt.
- Удалите фекалии и замените 10% морской воды (20 ppt) ежедневно.
- Вскрытие
- Очистите лоток для рассечения, ножницы и щипцы 70% этанолом.
- Анестезируйте самок индивидуально методом иммерсионной анестезии 2-PE.
- Случайным образом отбирают только что созревших самок (после линьки недоношенных самок), которые не прошли абляцию глазных стебельков, чтобы подтвердить их гонадные стадии.
- Пожертвуйте всеми подопытными самками с удаленными глазными стеблями по отдельности и определите стадии созревания гонад. Разрушают грудные ганглии краба с помощью острого стерильного шила. Сначала удалите верхний панцирь, а затем гепатопанкреа, чтобы сделать завязь видимой. Понаблюдайте за цветом яичников и определите стадию созревания яичников (рис. 2).
- Определение стадий созревания яичников
- Наблюдайте за цветом яичников невооруженным глазом или под стереомикроскопом.
- Определите стадии созревания яичников на основе окраски30: незрелая (стадия-1) имеет полупрозрачный или кремово-белый цвет; раннеспелый (стадия-2) имеет бледно-светло-желтоватый цвет; (iii) предварительное созревание (стадия-3) имеет цвет от желтого до светло-оранжевого; и (iv) полностью созревший (стадия 4) имеет цвет от темно-оранжевого до красноватого.
Результаты
Созревание гонад
Кремово-белые ткани яичников (незрелые яичники, стадия-1) были обнаружены у 100% рассеченных самок (n = 6) до проведения абляции глазных стебельков (рис. 2). Скорость созревания гонад у самок крабов с удаленным глазным стеблем (n = 63; 31 самка с техникой...
Обсуждение
Этот протокол был разработан для абляции глазного стебля грязевого краба Scylla spp. и может применяться в качестве эффективного метода для стимуляции созревания гонад. Этот протокол может быть легко воспроизведен для коммерческого созревания яичников грязевых крабов и может быть ре...
Раскрытие информации
Ни у кого из авторов нет конфликта интересов.
Благодарности
Это исследование было поддержано Министерством образования Малайзии в рамках программы Центра передового опыта высших учебных заведений (HICoE), Малайзия, аккредитованной при Институте тропической аквакультуры и рыболовства, Университет Малайзии Теренггану (Vot No 63933 и Vot No 56048). Мы выражаем признательность за поддержку Universiti Malaysia Terengganu и Sayap Jaya Sdn. Bhd. в рамках исследовательского гранта частного партнерства (Vot. No 55377). Также признана должность адъюнкт-научного сотрудника Университета Сайнс Малайзии для Хор Вайхо и Ханафии Фажан.
Материалы
| Name | Company | Catalog Number | Comments |
| Aeration tube | Ming Yu Three | N/A | aquarium and pet shop |
| Airstone | Ming Yu Three | N/A | aquarium and pet shop |
| Autoclave machine | HIRAYAMA MANUFACTURING CORPORATION | N/A | MADE IN JAPAN |
| Bleaching powder (Hi-Chlon 70%) | Nippon Soda Co.Ltd,Japan | N/A | N/A |
| Blow torch | MR D.I.Y. Group Berhad | N/A | N/A |
| Circular tank (32L) | BEST PLASTIC INDUSTRY SDN. BHD. | N/A | N/A |
| Cotton hand gloves (thick) | MR D.I.Y. Group Berhad | N/A | N/A |
| Cotton towel | MR D.I.Y. Group Berhad | N/A | N/A |
| Digital thermometer | Hanna Instrument | HI9814 | Hanna Instruments GroLine Hydroponics Waterproof pH / EC / TDS / Temp. Portable Meter HI9814 |
| Digital Vernier Caliper | INSIZE Co., Ltd. | N/A | |
| Dissecting tray | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Dropper bottle/Plastic Pipettes Dropper | Shopee Malaysia | N/A | N/A |
| Ethanol 70% | Thermo Scientific Chemicals | 033361.M1 | Diluted to 70% using double distilled water |
| Fiberglass tank (1 ton) | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Fine sand | N/A | N/A | collected from Sea beach of Universiti Malaysia Terengganu |
| First Aid Kits | Watsons Malaysia | N/A | N/A |
| Flat head nickel steel metal rod (Screw driver) | MR D.I.Y. Group Berhad | N/A | N/A |
| Formaldehyde | Thermo Scientific Chemicals | 119690010 | |
| Gas cylinder (butane gas) for blow torch | MR D.I.Y. Group Berhad | N/A | N/A |
| Gas lighter gun (long head) | MR D.I.Y. Group Berhad | N/A | N/A |
| Glass beaker (100 mL)) | Corning Life Sciences | 1000-100 | |
| Ice bag | Watsons Malaysia | N/A | N/A |
| Perforated plastic baskets | Eco-Shop Marketing Sdn. Bhd. | N/A | N/A |
| PVC pipe 15mm | Bina Plastic Industries Sdn Bhd (HQ) | N/A | N/A |
| Refractometer | ATAGO CO.,LTD. | ||
| Refrigerator | Sharp Corporation Japan | N/A | Chest Freezer SHARP 110L - SJC 118 |
| Scoop net | MR D.I.Y. Group Berhad | N/A | |
| Seawater | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Siphoning pipe | MR D.I.Y. Group Berhad | N/A | N/A |
| Spray bottle | Mr. DIY Sdn Bhd | N/A | N/A |
| Stainless surgical forceps | N/A | N/A | N/A |
| Stainless surgical scissors | N/A | N/A | N/A |
| Submersible water pump | AS | N/A | model: Astro 4000 |
| Tincture of iodine solution (Povidone Iodine) | Farmasi Fajr Sdn Bhd | N/A | N/A |
| Tissue paper | N/A | N/A | |
| Transparent plastic aquarium | Ming Yu Three | N/A | aquarium and pet shop |
| Waterproof table | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
Ссылки
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H., et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K., et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y., et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production. Aquaculture Reports. 18, 100432 (2020).
- Waiho, K., et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R., et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T., et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S., et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G., et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I., et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N., et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N., et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C., et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H. -. Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q., et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A., et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8, (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K., et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus. Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S. -. K., Lee, S. -. G., Lee, J. -. M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Archibald, K. E., Scott, G. N., Bailey, K. M., Harms, C. A. 2-phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). Journal of Zoo and Wildlife Medicine. 50 (1), 96-106 (2019).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity. Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X., et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C., et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C., et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Diarte-Plata, G., Sainz-Hernandez, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., Puente-Palazuelos, C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 130 (3-4), 172-178 (2012).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H., et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
Erratum
Formal Correction: Erratum: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs
Posted by JoVE Editors on 5/26/2023. Citeable Link.
An erratum was issued for: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs. The Introduction, Protocol, Discussion and References were updated.
The forth sentence in the third paragraph of the Introduction has been updated from:
The eyestalk ablation protocol in this work minimizes stress by using fully sedated crabs and minimizes physical injury to personnel from crab bites.
to:
The eyestalk ablation protocol in this work minimizes stress by using fully anesthetized crabs and minimizes physical injury to personnel from crab bites.
The start of the Protocol has been updated from:
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia. The sacrifice of the experimental samples was done according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Sexually pre-mature female mud crabs (orange mud crab S. olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
to:
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia and was approved by the Universiti Malaysia Terengganu's Research Ethics Committee (Animal ethics approval number: UMT/JKEPHMK/2023/96). The sacrifice of the experimental samples was done according to the AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Sexually pre-mature female mud crabs (orange mud crab Scylla olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
Section 4 of the Protocol has been updated from:
4. Cold-shock anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for cold shock anesthesia.
- Prepare 2 L of 4 °C to 1 °C seawater (20 ppt) in a transparent plastic aquarium. Maintain the temperature using (−20 °C) ice bags for cold shock anesthesia.
NOTE: Check the temperature with a digital thermometer. - Immerse the crab in the 4 °C seawater until sedated (about 3−5 min).
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement. The legs and chelipeds joints will still show minor movements when touched with forceps.
to:
4. Anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for anesthesia.
- After 5 min of acclimatization period, add 2-phenoxyethanol (2-PE) at 2 mL/L into each aquarium and allow 15 min of anesthesia treatment.
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement.
Section 5 of the Protocol has been updated from:
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized (sedated) crab with a wet cotton towel.
NOTE: Cover all the tentacles of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
- After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Prepare the tincture of iodine solution in a dropper bottle.
NOTE: Tincture of iodine (iodine tincture or weak iodine solution) is made up of 2%-7% elemental iodine and potassium iodide, or sodium iodide, dissolved in ethanol and water. - Wear thick cotton gloves.
- Hold the sedated crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
- Apply two to three drops of iodine tincture to the wounded part of the eyestalk immediately after cutting it off.
NOTE: Tincture of iodine is used for healing and to prevent infection.
to:
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle and keep it away from any fire-related sources, such as blow torch and red hot screwdriver. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized crab with a wet cotton towel.
NOTE: Cover the antennae of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color. Be careful when conducting this step to avoid damage to adjacent structures.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
NOTE: Only perform this step at least waiting for 5 min after the eyestalk ablation procedure to ensure the forceps are cooled down before disinfection using 70% ethanol to prevent potential fire hazards. - After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Wear thick cotton gloves.
- Hold the anesthetized crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
Step 7.2.2 of the Protocol has been updated from:
Sedate the females individually with the cold shock anesthesia method.
to:
Anesthetize the females individually with the 2-PE immersion anesthesia method.
The Discussion has been updated from:
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-of-season spawning11,12,13. Eyestalk ablation in brachyuran crabs has also been done to study molting25,32,33, hormonal regulation18, gonad maturation34, and induced breeding and reproductive performance35,36,37,38,39. Unilateral or bilateral eyestalk ablation influences the physiology of the crustacean. Eyestalk ablation following the protocol stated in this study also influences the ovarian maturation rate of mud crabs. In the control treatment (without eyestalk ablation), 43.33% ± 5.77% of female crabs had an immature ovary (stage-1). However, in the same rearing period (30 days), eyestalk-ablated female crabs had pre-maturing ovaries (stage-3; 56.67% ± 11.55% and 53.33% ± 15.28% with the cauterization and surgery techniques, respectively), which shows that eyestalk ablation can increase the gonad maturation of mud crabs. Previous studies have also reported that the ovarian development of intact crabs (without eyestalk ablation) is slower than that of eyestalk-ablated crabs25,31. Due to the slower gonadal development in intact crustaceans, eyestalk ablation is widely done in commercial prawn and shrimp hatcheries. In this protocol, the eyestalk-ablated female crabs achieved higher percentages of ovarian maturation compared to the female crabs without the eyestalk ablation treatment (Figure 3).
The gonad maturation of the mud crab is regulated by hormones21,40,41. The eyestalk contains important endocrine glands (i.e., the X-organ-sinus gland complex) that play vital roles in the gonadal maturation process of mud crabs18,21. Unilateral eyestalk ablation, either by cauterization or surgery, damages one of the major endocrine glands that is involved in the synthesis and release of inhibiting hormones (e.g., VIH), thereby resulting in a higher level of gonad-stimulating hormones (i.e., VSH).
The ovarian maturation stages of Scylla spp. can be differentiated by observing the ovarian tissue coloration with the naked eye29,30,42. Translucent or creamy white ovarian tissues are indications of immature ovaries29,30,42,43. In this study, immature ovaries (stage-1) were still found in the group of female crabs without eyestalk ablation due to the slower ovarian maturation process. However, the crabs in the eyestalk-ablated groups (both by the cauterization and surgery techniques) mostly showed pre-maturing ovaries (stage-3), with some individuals exhibiting fully matured ovaries (stage-4). Therefore, the protocol of eyestalk ablation described here can be used to increase ovarian maturation in female mud crabs. This protocol can also be applied directly to wild-collected mature female mud crabs to hasten their seed production. To evaluate the effectiveness of cauterization and surgery methods on mud crab gonad maturation and to ensure the accurate estimation of molting duration, sexually pre-mature crabs were used. After the (induced) molting of sexually pre-mature female crabs, we noticed that their ovaries were still in the immature or early developing stages29,44. After 30 days of rearing the newly mature female crabs (either eyestalk-ablated or without eyestalk ablation), the ovarian development stages (stage-1 to stage-4) were determined by the color of the ovarian tissues. This protocol encourages the use of the cauterization technique to perform eyestalk ablation in mud crabs to avoid any hemolymph loss and prevent infection at the ablated sites. Cauterization immediately seals the wound, whereas the surgery technique requires an additional step of disinfection using iodine. For commercial purposes, larger mature crabs, preferably at a later stage of ovarian maturation, should be selected for eyestalk ablation to shorten the time to reach the fully matured ovary stage for subsequent commerce or brood stock culture. In addition to eyestalk ablation, individual rearing with sand substrate and sufficient feeding, preferably with live feed, can increase the gonad maturation rate of mud crabs in captivity30,35,45,46.
Crustacean blood is called hemolymph and can be lost during eyestalk ablation. An excessive loss of hemolymph may lead to the death of eyestalk-ablated crabs, especially when performing surgery to remove the eyestalk. The hemolymph can coagulate in the wounded part to prevent loss. The application of a tincture of iodine can prevent infection of the wounded part. However, in comparison to the surgery technique, the cauterization technique seals the wounded part immediately, thereby preventing the loss of hemolymph and possible infection.
Mud crab mortality after unilateral eyestalk ablation with either cauterization or surgery was not found within the first 7 days. Thus, eyestalk ablation can be done with a higher survival rate. Unilateral eyestalk ablation does not hamper the survival rate of the crab33.
Stress during crab handling and eyestalk ablation may contribute to crab mortality. Proper anesthesia is needed to minimize handling stress during eyestalk ablation. In crustacean eyestalk ablation, chemical anesthetics (i.e., xylocaine, lidocaine) are used at the base of the eyestalk before eyestalk ablation14,15,17,47. However, due to the aggressive nature and large size of mud crabs, the use of anesthesia only at the base of the eyestalk is not sufficient and might result in additional stress to the animals during the injection. On the other hand, anesthesia by subjecting them to a lower water temperature is more economical and safer. The use of cold water for anesthesia in mud crabs is common and has been used in other studies due to its efficiency, simplicity, and minimal impact on recovery and survival37,48,49.
Although eyestalk ablation using both cauterization and surgery methods has a minimal effect on crab survival and enhances ovarian maturation, performing eyestalk ablation requires professional mastery of the techniques. The timing between the steps is critical as any delay between protocols adds additional stress for the crabs. Unlike the surgery technique, the cauterization technique is dangerous because it involves the use of flammable equipment (i.e., a blow torch and butane gas). Thus, extra caution is needed when performing the cauterization technique.
Crabs are cannibalistic in nature, and they are known to prey on others that have just completed their molt and are still in their soft-shell conditions7,50,51. Thus, rearing the crabs individually can avoid unnecessary mortality due to cannibalism. The use of individual rearing in mud crab culture is commonly practiced, both in high-density culture and pond culture, for fattening and soft-shell crab farming purposes8,52. This protocol also utilized individual rearing and maintenance. During the transportation of the crabs for rearing or commerce, the crab chelipeds are tied up securely (or even autotomized) to prevent fighting, unnecessary injury, and limb loss34.
The described protocol for eyestalk ablation should be performed with multiple persons. After completing the eyestalk ablation, non-disposable equipment (e.g., the aquarium, tray, towel, etc.) should be disinfected with 30 ppm chlorine. The crabs must be monitored at least twice per day. Any dead crabs, uneaten feed, ablated limbs, or molted crab shells should be swiftly disposed of (i.e., buried in soil with bleaching powder) to prevent any potential for disease spread.
to:
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-of-season spawning11,12,13. Eyestalk ablation in brachyuran crabs has also been done to study molting25,32,33, hormonal regulation18, gonad maturation34, and induced breeding and reproductive performance35,36,37,38,39. Anesthesia via immersion in 2-phenoxyethanol was used as it is comparable to the use of tricaine methanesulfonate (MS-222) in arthopods but cheaper and does not require the use of additional buffer40. Unilateral or bilateral eyestalk ablation influences the physiology of the crustacean. Eyestalk ablation following the protocol stated in this study also influences the ovarian maturation rate of mud crabs. In the control treatment (without eyestalk ablation), 43.33% ± 5.77% of female crabs had an immature ovary (stage-1). However, in the same rearing period (30 days), eyestalk-ablated female crabs had pre-maturing ovaries (stage-3; 56.67% ± 11.55% and 53.33% ± 15.28% with the cauterization and surgery techniques, respectively), which shows that eyestalk ablation can increase the gonad maturation of mud crabs. Previous studies have also reported that the ovarian development of intact crabs (without eyestalk ablation) is slower than that of eyestalk-ablated crabs25,31. Due to the slower gonadal development in intact crustaceans, eyestalk ablation is widely done in commercial prawn and shrimp hatcheries. In this protocol, the eyestalk-ablated female crabs achieved higher percentages of ovarian maturation compared to the female crabs without the eyestalk ablation treatment (Figure 3).
The gonad maturation of the mud crab is regulated by hormones21,41,42. The eyestalk contains important endocrine glands (i.e., the X-organ-sinus gland complex) that play vital roles in the gonadal maturation process of mud crabs18,21. Unilateral eyestalk ablation, either by cauterization or surgery, damages one of the major endocrine glands that is involved in the synthesis and release of inhibiting hormones (e.g., VIH), thereby resulting in a higher level of gonad-stimulating hormones (i.e., VSH).
The ovarian maturation stages of Scylla spp. can be differentiated by observing the ovarian tissue coloration with the naked eye29,30,43. Translucent or creamy white ovarian tissues are indications of immature ovaries29,30,43,44. In this study, immature ovaries (stage-1) were still found in the group of female crabs without eyestalk ablation due to the slower ovarian maturation process. However, the crabs in the eyestalk-ablated groups (both by the cauterization and surgery techniques) mostly showed pre-maturing ovaries (stage-3), with some individuals exhibiting fully matured ovaries (stage-4). Therefore, the protocol of eyestalk ablation described here can be used to increase ovarian maturation in female mud crabs. This protocol can also be applied directly to wild-collected mature female mud crabs to hasten their seed production. To evaluate the effectiveness of cauterization and surgery methods on mud crab gonad maturation and to ensure the accurate estimation of molting duration, sexually pre-mature crabs were used. After the (induced) molting of sexually pre-mature female crabs, we noticed that their ovaries were still in the immature or early developing stages29,45. After 30 days of rearing the newly mature female crabs (either eyestalk-ablated or without eyestalk ablation), the ovarian development stages (stage-1 to stage-4) were determined by the color of the ovarian tissues. This protocol encourages the use of the cauterization technique to perform eyestalk ablation in mud crabs to avoid any hemolymph loss and prevent infection at the ablated sites. Cauterization immediately seals the wound, whereas the surgery technique takes time for the wound to heal and this would allow for chance of infection. For commercial purposes, larger mature crabs, preferably at a later stage of ovarian maturation, should be selected for eyestalk ablation to shorten the time to reach the fully matured ovary stage for subsequent commerce or brood stock culture. In addition to eyestalk ablation, individual rearing with sand substrate and sufficient feeding, preferably with live feed, can increase the gonad maturation rate of mud crabs in captivity30,35,46,47.
Crustacean blood is called hemolymph and can be lost during eyestalk ablation. An excessive loss of hemolymph may lead to the death of eyestalk-ablated crabs, especially when performing surgery to remove the eyestalk. The hemolymph can coagulate in the wounded part to prevent loss. However, in comparison to the surgery technique, the cauterization technique seals the wounded part immediately, thereby preventing the loss of hemolymph and possible infection.
Mud crab mortality after unilateral eyestalk ablation with either cauterization or surgery was not found within the first 7 days. Thus, eyestalk ablation can be done with a higher survival rate. Unilateral eyestalk ablation does not hamper the survival rate of the crab33.
Stress during crab handling and eyestalk ablation may contribute to crab mortality. Proper anesthesia is needed to minimize handling stress during eyestalk ablation. In crustacean eyestalk ablation, chemical anesthetics (i.e., xylocaine, lidocaine) are used at the base of the eyestalk before eyestalk ablation14,15,17,48. However, due to the aggressive nature and large size of mud crabs, the use of anesthesia only at the base of the eyestalk is not sufficient and might result in additional stress to the animals during the injection. On the other hand, anesthesia by subjecting them to a lower water temperature is more economical and safer. The use of cold water for anesthesia in mud crabs is common and has been used in other studies due to its efficiency, simplicity, and minimal impact on recovery and survival37,49,50. In addition, future research on pain assessment following eyestalk ablation on mud crabs is recommended to highlight the change in behaviours associated with pain and stress, as evident in freshwater prawn Macrobrachium americanum51.
Although eyestalk ablation using both cauterization and surgery methods has a minimal effect on crab survival and enhances ovarian maturation, performing eyestalk ablation requires professional mastery of the techniques. The timing between the steps is critical as any delay between protocols adds additional stress for the crabs. Unlike the surgery technique, the cauterization technique is dangerous because it involves the use of flammable equipment (i.e., a blow torch and butane gas). Thus, extra caution is needed when performing the cauterization technique.
Crabs are cannibalistic in nature, and they are known to prey on others that have just completed their molt and are still in their soft-shell conditions7,52,53. Thus, rearing the crabs individually can avoid unnecessary mortality due to cannibalism. The use of individual rearing in mud crab culture is commonly practiced, both in high-density culture and pond culture, for fattening and soft-shell crab farming purposes8,53. This protocol also utilized individual rearing and maintenance. During the transportation of the crabs for rearing or commerce, the crab chelipeds are tied up securely (or even autotomized) to prevent fighting, unnecessary injury, and limb loss34.
The described protocol for eyestalk ablation should be performed with multiple persons. After completing the eyestalk ablation, non-disposable equipment (e.g., the aquarium, tray, towel, etc.) should be disinfected with 30 ppm chlorine. The crabs must be monitored at least twice per day. Any dead crabs, uneaten feed, ablated limbs, or molted crab shells should be swiftly disposed of (i.e., buried in soil with bleaching powder) to prevent any potential for disease spread.
The References have been updated from:
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H. et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters, Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K. et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y. et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production? Aquaculture Reports. 18, 100432 (2020).
- Waiho, K. et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R. et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T. et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S. et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G. et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I. et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N. et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N. et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C. et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H.-Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q. et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A. et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8 (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K. et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus.Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S.-K., Lee, S.-G., Lee, J.-M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity? Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X. et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C. et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C. et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H. et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
to:
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H. et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters, Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K. et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y. et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production? Aquaculture Reports. 18, 100432 (2020).
- Waiho, K. et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R. et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T. et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S. et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G. et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I. et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N. et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N. et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C. et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H.-Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q. et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A. et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8 (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K. et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus.Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S.-K., Lee, S.-G., Lee, J.-M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Archibald, K. E., Scott, G. N., Bailey, K. M., Harms, C. A. 2-phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). Journal of Zoo and Wildlife Medicine. 50 (1), 96-106 (2019).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity? Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X. et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C. et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C. et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Diarte-Plata, G., Sainz-Hernandez, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., Puente-Palazuelos, C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H. et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
Перепечатки и разрешения
Запросить разрешение на использование текста или рисунков этого JoVE статьи
Запросить разрешениеСмотреть дополнительные статьи
This article has been published
Video Coming Soon
Авторские права © 2025 MyJoVE Corporation. Все права защищены