Some solids can transition directly into the gaseous state, bypassing the liquid state, via a process known as sublimation. At room temperature and standard pressure, a piece of dry ice (solid CO2) sublimes, appearing to gradually disappear without ever forming any liquid. Snow and ice sublimate at temperatures below the melting point of water, a slow process that may be accelerated by winds and the reduced atmospheric pressures at high altitudes. When solid iodine is warmed, the solid sublimes and a vivid purple vapor forms. The reverse of sublimation is called deposition, a process in which gaseous substances condense directly in the solid-state, bypassing the liquid state. The formation of frost is an example of deposition.
Like vaporization, the process of sublimation requires an input of energy to overcome intermolecular attractions. Sublimation is, therefore, an endothermic phase transition. The enthalpy of sublimation, ΔHsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. For example, the sublimation of carbon dioxide is represented by:

Likewise, the enthalpy change for the reverse process of deposition is equal in magnitude but opposite in sign to that for sublimation. Because deposition involves the formation of intermolecular forces, it is an exothermic phase transition.

Consider the extent to which intermolecular attractions must be overcome to achieve a given phase transition. Converting a solid into a liquid requires that these attractions be only partially overcome; transition to the gaseous state requires that they be completely overcome. As a result, the enthalpy of fusion for a substance is less than its enthalpy of vaporization. This same logic can be used to derive an approximate relation between the enthalpies of all phase changes for a given substance. Though not an entirely accurate description, sublimation may be conveniently modeled as a sequential two-step process of melting followed by vaporization in order to apply Hess’s Law. Viewed in this manner, the enthalpy of sublimation for a substance may be estimated as the sum of its enthalpies of fusion and vaporization.
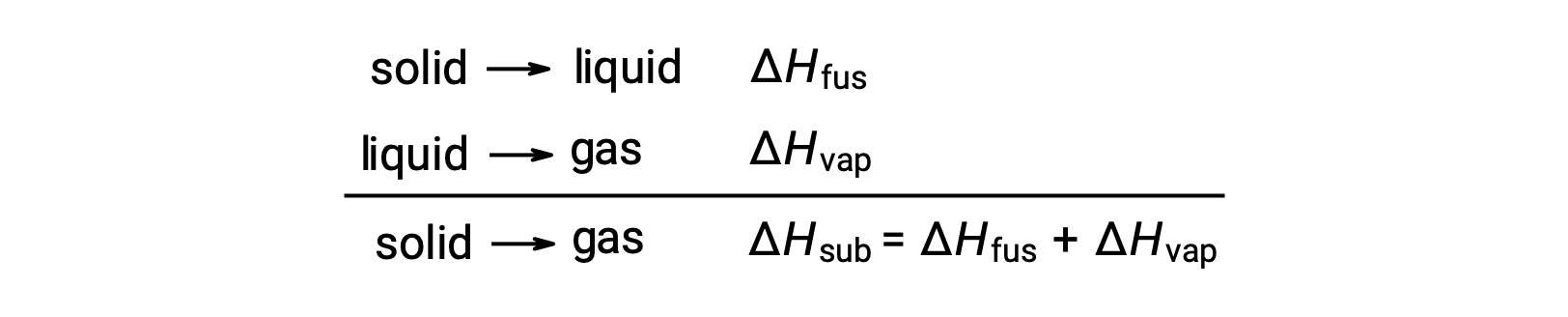
This text is adapted from Openstax, Chemistry 2e, Section 10.3: Phase Transitions.
From Chapter 11:

Now Playing
11.11 : Phase Transitions: Sublimation and Deposition
Liquids, Solids, and Intermolecular Forces
16.5K Views

11.1 : Molecular Comparison of Gases, Liquids, and Solids
Liquids, Solids, and Intermolecular Forces
40.0K Views

11.2 : Intermolecular vs Intramolecular Forces
Liquids, Solids, and Intermolecular Forces
84.1K Views

11.3 : Intermolecular Forces
Liquids, Solids, and Intermolecular Forces
55.4K Views

11.4 : Comparing Intermolecular Forces: Melting Point, Boiling Point, and Miscibility
Liquids, Solids, and Intermolecular Forces
43.4K Views

11.5 : Surface Tension, Capillary Action, and Viscosity
Liquids, Solids, and Intermolecular Forces
27.2K Views

11.6 : Phase Transitions
Liquids, Solids, and Intermolecular Forces
18.5K Views

11.7 : Phase Transitions: Vaporization and Condensation
Liquids, Solids, and Intermolecular Forces
16.9K Views

11.8 : Vapor Pressure
Liquids, Solids, and Intermolecular Forces
33.9K Views

11.9 : Clausius-Clapeyron Equation
Liquids, Solids, and Intermolecular Forces
54.9K Views

11.10 : Phase Transitions: Melting and Freezing
Liquids, Solids, and Intermolecular Forces
12.1K Views

11.12 : Heating and Cooling Curves
Liquids, Solids, and Intermolecular Forces
22.1K Views

11.13 : Phase Diagrams
Liquids, Solids, and Intermolecular Forces
38.6K Views

11.14 : Structures of Solids
Liquids, Solids, and Intermolecular Forces
13.5K Views

11.15 : Molecular and Ionic Solids
Liquids, Solids, and Intermolecular Forces
16.5K Views
See More
Copyright © 2025 MyJoVE Corporation. All rights reserved