A balanced chemical equation provides the information of chemical formulas of the reactants and products involved in the chemical change. A reaction’s stoichiometry helps predict how much of the reactant is needed to produce the desired amount of product, or in some cases, how much product will be formed from a specific amount of the reactant.
The relative amounts of reactants and products represented in a balanced chemical equation are often referred to as stoichiometric amounts. However, in reality, the reactants are not always present in the stoichiometric amounts indicated by the balanced equation.
In a chemical reaction, the reactant which gets consumed first, and limits the amount of product formed, is the limiting reactant, while the other substance becomes the excess reactant. An excess of one or more reactants is often used to ensure the complete conversion of the other reactant into the product.
Consider the reaction for the formation of water represented by the equation:
Stoichiometry indicates that two moles of hydrogen and one mole of oxygen react to produce two moles of water; that is, hydrogen and oxygen combine in a 2:1 ratio.
Imagine if 5 moles of hydrogen and 2 moles of oxygen are present. The ratio of the reactants is now 5:2 (or 2.5:1), which is greater than the stoichiometric ratio of 2:1. Hydrogen, therefore, is present in excess, and oxygen is the limiting reactant. Reaction of all the provided oxygen (2 mol) will consume 4 mol of the 5 mol of hydrogen provided, leaving 1 mol of hydrogen unreacted. Computing the molar amounts of each reactant provided and comparing them to the stoichiometric amounts represented in the balanced chemical equation is one way of identifying the limiting and excess reactants.
The rate of reaction is the change in the amount of a reactant or product per unit time. Reaction rates are therefore determined by measuring the time dependence of some property that can be related to reactant or product amounts.
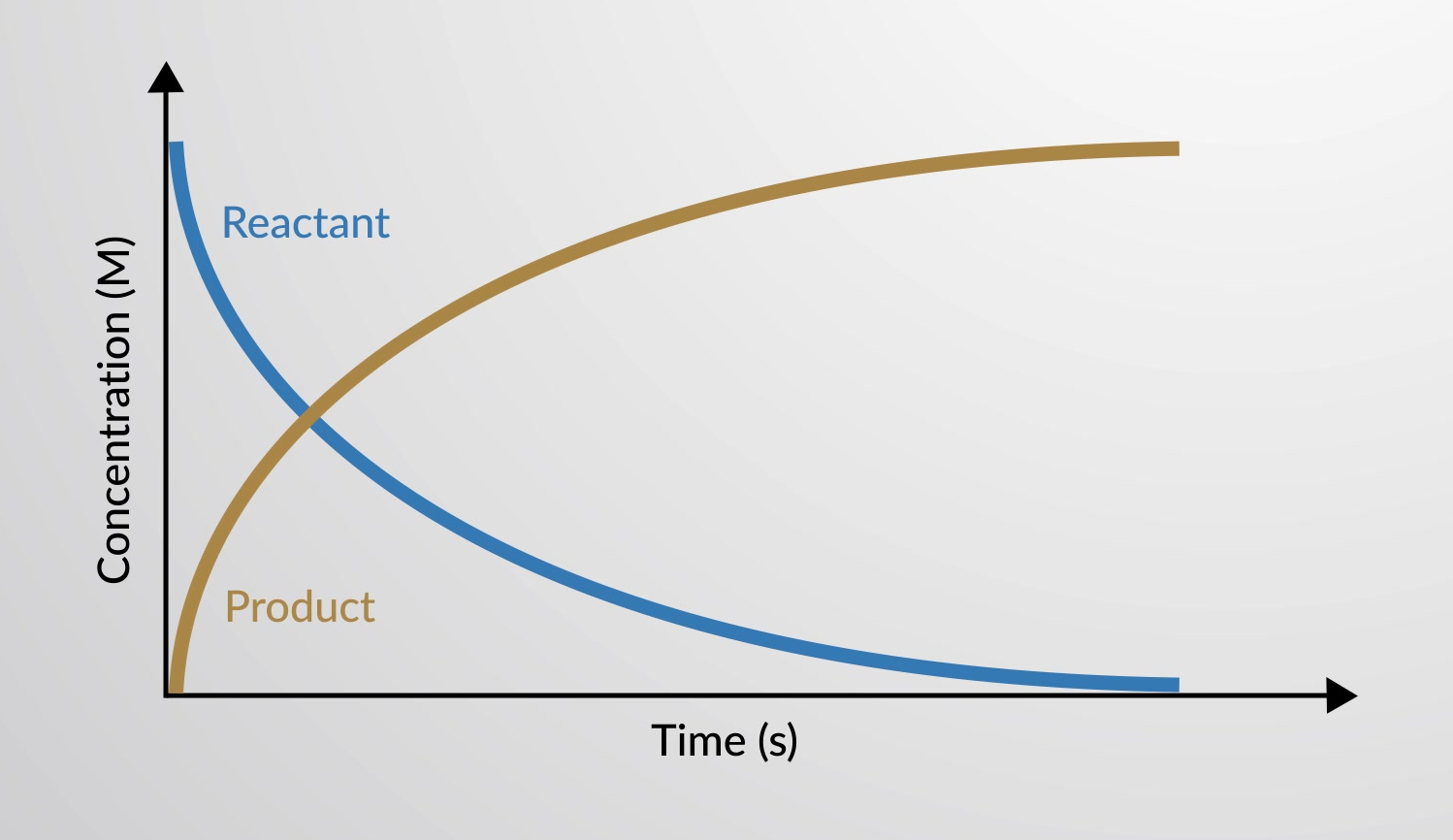
The rate of a chemical reaction can be plotted in a graph as the variance in concentrations of reactants and products as a function of time.
A reversible chemical reaction represents a chemical process that proceeds in both forward (left to right) and reverse (right to left) directions. The status of a reversible reaction is conveniently assessed by evaluating its reaction quotient (Q). For a reversible reaction described by
the reaction quotient is derived directly from the stoichiometry of the balanced equation as
where the subscript c denotes the use of molar concentrations in the expression.
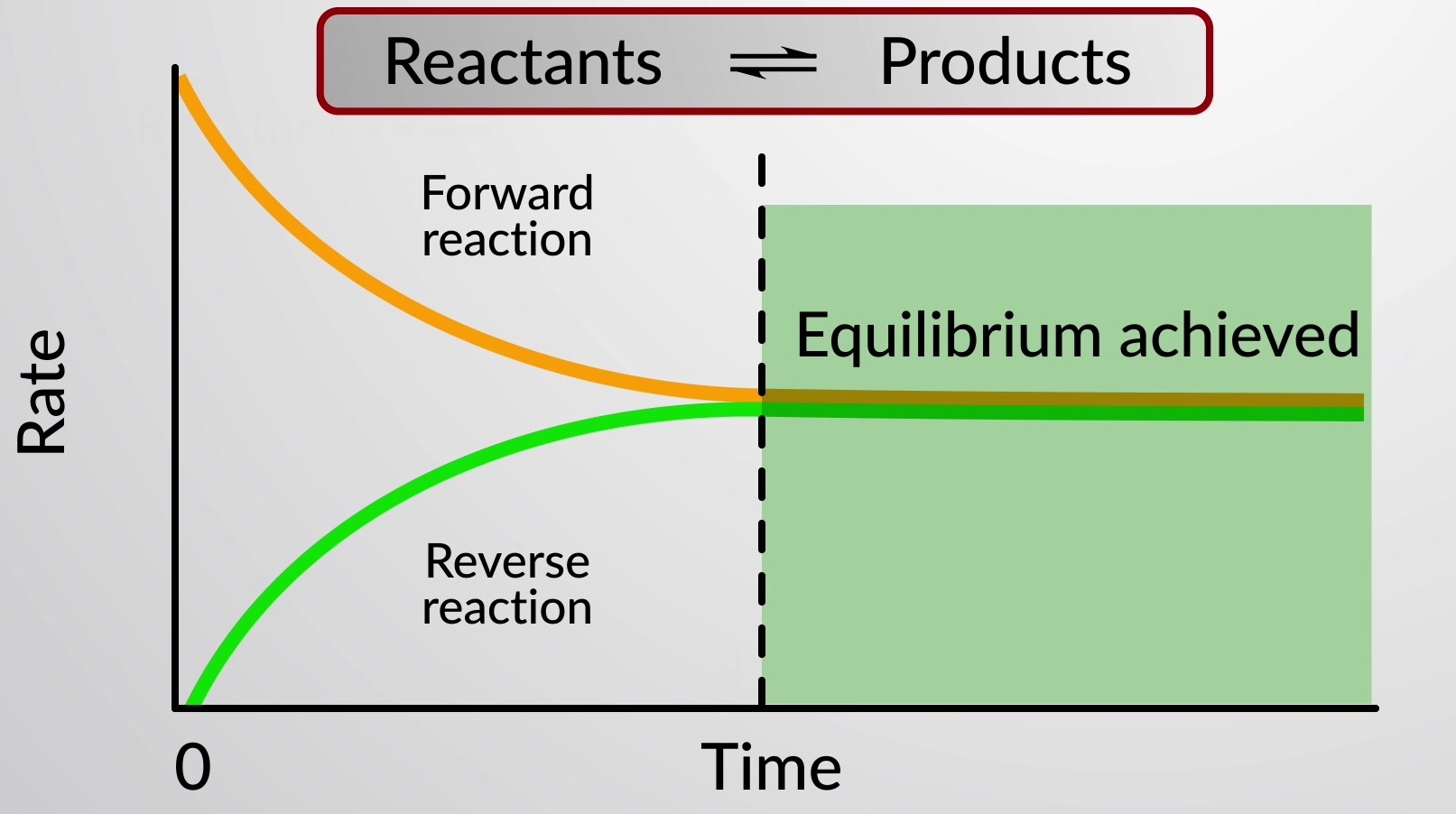
When the rates of the forward and reverse reactions are equal, the concentrations of the reactant and product species remain constant over time and the system is at equilibrium. A special double arrow is used to emphasize the reversible nature of the reaction.
This text is adapted fromOpenStax Chemistry 2e, Section 4.3: Reaction Stoichiometry;Section 4.4: Reaction Yield;Section 12.1: Chemical Reaction Rates;Section 13.1 Chemical Equilibria,Section 13.2 Equilibrium Constants.
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved