Purification of Ferrocene by Sublimation
Source: Tamara M. Powers, Department of Chemistry, Texas A&M University
Sublimation, the direct phase transition of a solid into a gas without first becoming a liquid, takes place at temperatures and pressures lower than that of the compound's triple point (Figure 1).The process of sublimation can be utilized to purify both organic and inorganic solids. During the purification technique, a solid is heated directly into the gas-phase. All non-volatile impurities are left behind while the vaporized compound is then collected (deposition) as a solid on a cold surface. Here, we will use sublimation to purify ferrocene, an inorganic solid with a triple point temperature of 183 °C.1
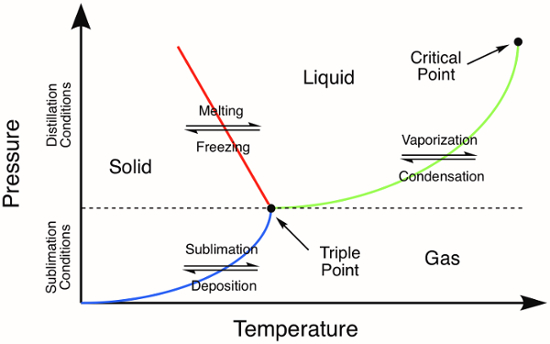
Figure 1. Generic phase diagram. The colored lines represent the pressure and temperature requirements for phase transitions. Distillation of a solid will occur at pressures and temperatures above the triple point, represented by the green line in the phase diagram. The blue line represents the temperature and pressure conditions where sublimation occurs.
1. Setup of the Schlenk Line
For a more detailed procedure, please review the "Schlenk Lines Transfer of Solvent" and "Degassing Liquids" videos in the Essentials of Organic Chemistry series. Schlenk line safety should be reviewed prior to conducting this experiment. Glassware should be inspected for star cracks before use. Care should be taken to ensure that O2 is not condensed in the Schlenk line trap if using liquid N2. At liquid N2 te
Ferrocene (99%) was purchased from Alfa Aesar. Sublimation of 500 mg as described resulted in 493 mg isolated product. The purified ferrocene was analyzed by 1H NMR. 1H NMR (chloroform-d, 300 MHz, δ, ppm): 4.17 (s).
Sublimation is a technique used in the purification of solids. Solids that sublime at low pressure and temperature are good candidates for purification by sublimation. Here, we have demonstrated how to use a sublimation chamber to sublime ferrocene under static vacuum at 80 °C.
In a laboratory setting, sublimation is a useful technique that can be applied to the purification of solids in a variety of situations including in the purification of starting materials or synthesized products. I
- Kaplan, L., Kester, W. L., Katz, J. J. Some properties of iron biscyclopentadienyl. J Am Chem Soc. 74, 5531-5532 (1952).
Skip to...
Videos from this collection:

Now Playing
Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.1K Views

Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.4K Views

Glovebox and Impurity Sensors
Inorganic Chemistry
18.5K Views

The Evans Method
Inorganic Chemistry
67.3K Views

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
103.2K Views

Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.3K Views

Mössbauer Spectroscopy
Inorganic Chemistry
21.9K Views

Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
38.7K Views

Structure Of Ferrocene
Inorganic Chemistry
78.4K Views

Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
44.8K Views

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.0K Views

Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.2K Views

Dye-sensitized Solar Cells
Inorganic Chemistry
15.4K Views

Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.1K Views

Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
16.6K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved