Photochemical Initiation Of Radical Polymerization Reactions
Overview
Source: David C. Powers, Tamara M. Powers, Texas A&M
In this video, we will carry out the photochemically initiated polymerization of styrene to generate polystyrene, which is an important commodity plastic. We will learn the fundamentals of photochemistry and use simple photochemistry to initiate radical polymerization reactions. Specifically, in this module we will examine the photochemistry of benzoyl peroxide and its role as a photo-initiator of styrene polymerization reactions. In the described experiments, we will investigate the role of wavelength, photon absorption, and excited state structure on the efficiency (measured as quantum yield) of photochemical reactions.
Principles
Organic polymers are ubiquitous chemicals in everyday life. Polyolefins, which are generated by the polymerization of alkene monomers, comprise common plastics and rubbers found in drinking cups, soda bottles, car tires, and even some fabrics. Polystyrene, for example, is a polymer based on styrene monomers, and finds important applications in protective packaging (i.e., packing peanuts), water bottles, and disposable forks and knives. Polystyrene is generated on a scale of several million tons per year.
Polymer synthesis is a field of chemistry devoted to developing methods to initiate and control polymer growth in order to generate polymers with specific targeted applications. For example, the exact properties of polyolefin materials depend intimately on the polymer chain structure, and structural factors such as the extent of chain branching can completely change the properties of polymeric materials built from the same monomer units.
Simple olefins do not spontaneously participate in polymerization chemistry, despite the fact that these reactions are thermodynamically downhill. To get efficient polymerization to proceed, either initiators or catalysts, which lower the kinetic barrier to polymerization, are required. Benzoyl peroxide is an example of an effective radical initiator for polymer growth. Photochemically promoted homolytic cleavage of the O-O bond results initially in the generation of carboxylate radicals and, following decarboxylation, in phenyl radicals and CO2 (Figure 1). These phenyl radicals react with styrene to generate a new C-C bond and a benzylic radical. This initially formed benzylic radical can react with additional styrene in a radical chain reaction to eventually afford long-chains of polystyrene.
The basis of photochemistry is that photon absorption generates an excited state in molecules, which increases the likelihood of molecular participation in chemical reactions. In order to access the required state of molecular excitation, the molecule of interest (in this case, benzoyl peroxide) needs to have absorbed light at a specific wavelength. Benzoyl peroxide absorbs only in the UV portion of the spectrum; O-O homolysis can be initiated by irradiation with ~250 nm light. This wavelength is too short for the human eye to see. To illustrate the importance of photon absorption for photochemistry, we will first examine the polymerization of styrene initiated by benzoyl peroxide directly under visible light irradiation. The absorption spectrum of benzoyl peroxide does not have significant absorption in the visible region (benzoyl peroxide is colorless), and photoinduced polymerization with visible light is not facile.
In order to overcome the poor absorptivity of many organic molecules towards visible light, photosensitizers are utilized. Photosensitizers are molecules that participate in photon absorption and then transfer energy to a different molecule to promote photochemical reactions. Benzophenone is a common photosensitizer; the photochemistry of this molecule is described in Figure 2. Photon absorption in the ground state generates a singlet excited state. Intersystem crossing affords the triplet ground state, which compared to the singlet excited state, is long lived. Energy transfer from the triplet excited state to benzoyl peroxide can lead to O-O bond cleavage and radical-chain initiation. The photosenstitizer is useful because unlike benzoyl peroxide, it has substantial absorption in the visible spectrum.
In competition with energy transfer to substrate, the triplet excited state can undergo relaxation to regenerate the singlet ground state; if this process is fast relative to energy transfer, then sensitization is not efficient. The efficiency of sensitization is measured by the quantum yield, which is a measure of the fraction of absorbed photons that are utilized productively in the targeted chemical reaction. In order for photosensitizers to work effectively, the excited state of the sensitizer must encounter the other reactant; in our case, the triplet excited state of benzophenone must encounter benzoylperoxide in order to accomplish energy transfer. The quantum yield of polymerization using bimolecular initiators is low if relatively few of the photogenerated benzophenone excited states result in generation of benzoyl radicals by O-O cleavage.
Quantum Yield = photoreactions accomplished / photons absorbed
Through the experiments that are outlined in this video, we will confront the topics of photochemistry, triplet sensitizers, and polymerization chemistry.
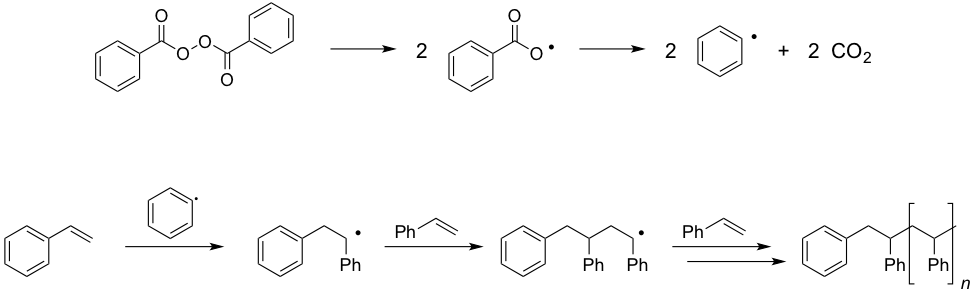
Figure 1. (a) O-O bond cleavage eventually leads to the formation of phenyl radicals, which can initiate polymerization. (b) Illustration of the radical-chain polymerization initiated by phenyl radicals.
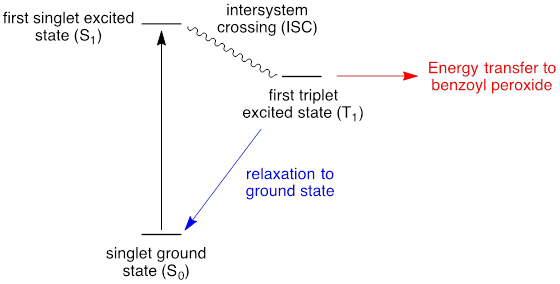
Figure 2. Diagram of photosensitization. Photon absorption by benzophenone initially generates the first singlet excited state (S1). Intersystem crossing provides access to the first triplet excited state (T1). Energy transfer from the triplet excited state to benzoyl peroxide leads to productive photochemistry.
Procedure
1. Measure the absorption spectrum of benzoyl peroxide.
- Benzoyl peroxide is commercially available. Prepare a solution of benzoyl peroxide in toluene. Using a 10 mL volumetric flask, add 10 mg of benzoyl peroxide. Fill the volumetric flask with toluene.
- Add 0.5 mL of the prepared solution to a UV-vis cuvette using a volumetric pipette. Add 3.5 mL of toluene.
- Prepare a second cuvette, filled only with pure toluene.
- Measure the absorption spectrum (300–800 nm wavelength range) of the toluene-filled cuvette. This spectrum should be used to subtract the solvent background from the spectrum of benzoyl peroxide collected below.
- Measure the absorption spectrum (300–800 nm wavelength range) of the benzoyl peroxide-containing cuvette. To obtain the absorption spectrum of benzoyl peroxide, subtract the spectrum of toluene obtained in step 1.4. Many UV-vis software packages perform background subtraction automatically. If not, background subtraction can be accomplished using standard database software, such as Excel.
2. Reaction of benzoyl peroxide and styrene in the absence of a photosensitizer.
- Measure the tare weight of a 25-mL round-bottom flask.
- Prepare a solution of benzoyl peroxide and styrene in toluene by combining 1 mL of the solution prepared above to 10 mL toluene and 3 mL styrene. Transfer the reaction solution to a 25-mL round-bottom flask, fit the flask with a rubber septum, and degas the reaction solution by bubbling nitrogen through the solution (see the “Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique” video in the Inorganic Chemistry series). It is not important to exclude water from the reaction solution, only to remove dissolved O2.
- In a ventilated fume hood, clamp the reaction flask fitted with the nitrogen filled balloon to a stir plate. Turn on the Hg-arc lamp and wait 10 minutes for the light bulb to warm up. With magnetic stirring, irradiate the solution with a Hg-arc lamp using a 350-nm long-pass filter for 10 minutes.
- Concentrate the photoreaction on a rotovap. If non-volatile residue remains, obtain a mass of the flask. The weight of the non-volatile residue can be determined using the tare weight measured in step 2.1 Obtain a 1H NMR spectrum of the residue in CDCl3.
3. Measure the absorption spectrum of benzophenone.
- Benzophenone is commercially available. Prepare a solution of benzophenone in toluene. Using a 10 mL volumetric flask, add 10 mg of benzophenone. Fill the volumetric flask with toluene.
- Add 0.5 mL of the prepared solution to a UV-vis cuvette using a volumetric pipette. Add 3.5 mL of toluene.
- Measure the absorption spectrum (300–800 nm wavelength range) of benzophenone.
- Using the spectrum of toluene obtained in Step 1.4, perform a background subtraction to obtain the absorption spectrum of pure benzophenone.
4. Reaction of benzoyl peroxide and styrene in the presence of the photosensitizer benzophenone.
- Measure the tare weight of a 25-mL round-bottom flask.
- Prepare a solution of benzoyl peroxide, benzophenone, and styrene in toluene by combining 1.0 mL of the benzoyl peroxide solution prepared in step 1.1, with 1.0 mL of the benzophenone solution prepared in step 3.1 with 10 mL toluene and 3 mL styrene. Transfer the reaction solution to a 25-mL round-bottom flask, fit the flask with a rubber septum, and degas the reaction solution by bubbling nitrogen through the solution (see the “Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique” video in the Inorganic Chemistry series). It is not important to exclude water from the reaction solution, only to remove dissolved O2.
- In a ventilated fume hood, clamp the reaction flask fitted with the nitrogen filled balloon to a stir plate. Turn on the Hg-arc lamp and wait 10 minutes for the light bulb to warm up. With magnetic stirring, irradiate the solution with a Hg-arc lamp using a 350-nm long-pass filter for 10 minutes.
- Concentrate the photoreaction on a rotovap. If non-volatile residue remains, obtain a mass of the flask. The weight of the non-volatile residue can be determined using the tare weight measured in step 4.1 Obtain a 1H NMR spectrum of the residue in CDCl3.
5. Control reaction of styrene in the presence of the photosensitizer benzophenone.
- Measure the tare weight of a 25-mL round-bottom flask.
- Prepare a solution of benzophenone and styrene in toluene by combining 1.0 mL of the benzophenone solution prepared in step 3.1 with 10 mL toluene and 3 mL styrene. Transfer the reaction solution to a 25-mL round-bottom flask, fit the flask with a rubber septum, and degas the reaction solution by bubbling nitrogen through the solution (see the “Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique” video in the Inorganic Chemistry series). It is not important to exclude water from the reaction solution, only to remove dissolved O2.
- In a ventilated fume hood, clamp the reaction flask fitted with the nitrogen filled balloon to a stir plate. Turn on the Hg-arc lamp and wait 10 minutes for the light bulb to warm up. With magnetic stirring, irradiate the solution with a Hg-arc lamp using a 350-nm long-pass filter for 10 minutes.
- Concentrate the photoreaction on a rotovap. If non-volatile residue remains, obtain a mass of the flask. The weight of the non-volatile residue can be determined using the tare weight measured in step 5.1 Obtain a 1H NMR spectrum of the residue in CDCl3.
Results
Based on the UV-vis absorption spectra that we collected, benzoyl peroxide does not display substantial absorption in the visible spectrum. The lack of visible-light-absorption is consistent with the lack of polymerization chemistry that is observed when a sample of styrene is photolyzed in the presence of benzoyl peroxide. The residue left behind following evaporation of the photoreaction described in step 2 contains only benzoyl peroxide; no styrene-derived products have been generated.
In contrast to benzoyl peroxide, benzophenone does absorb a substantial amount of visible light (> 300 nm). Photolysis of a mixture of benzophenone, benzoyl peroxide, and styrene results in the formation of some polystyrene. The polymerization results in the formation of an oily, non-volatile residue that remains after evaporation of the photoreaction. In addition, 1H NMR analysis of the residue indicates the presence of polystyrene. Polystyrene is characterized by diagnostic 1H NMR signals: the aromatic protons appear as a broad multiplet from 7.2-6.4 ppm and the aliphatic protons appear as multiplets centered at 1.9 and 1.5 ppm that integrate in a 1:2 ratio.
Finally, note that benzophenone alone is not a competent photoinitiator. Only when the sensitizer, initiator, and substrate were all present did polymerization proceed.
Application and Summary
In this video, we have seen the impact of structure on the reactivity of radical initiators for olefin polymerization chemistry. We have examined photochemical conditions that: 1) did not contain appropriate absorbers, 2) contained appropriate absorbers but not appropriate initiators, and 3) contained both initiator and absorber molecules. These systems highlight the role of photon absorption and the importance of quantum efficiency on photochemical reactions.
Radical initiators are important tools for the production of polymer materials. Photo-initiated polymerization reactions find applications in a variety of areas. For example, photochemically initiated polymerization chemistry can be used to make designer materials in which the monomer that is incorporated is changed on demand, which allows precise control over the molecular structure of the material that results (Figure 3).
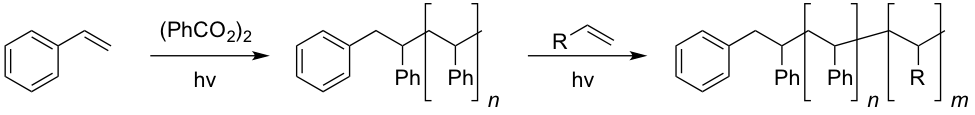
Figure 3. Photochemical control over block copolymer synthesis provides a strategy to making designer materials.
A second application is using photochemically initiated polymerization to generate 3-dimensionally patterned structures from polymers. Typically, such patterning is achieved by generating a mask, which prevents irradiation of areas of a surface that are covered with an appropriate monomer. Photoinduced polymerization is then carried out to generate a structure in which polymerization has been accomplished in the relief of the mask.
In this experiment, we saw the critical impact of photosensitizers on the efficiency of photochemical reactions. The concepts explored here underpin an important area of polymerization chemistry, which endeavours to identify highly efficient sensitizers and initiators. One example which we can understand based on our experiments is the use of unimolecular initiator-sensitizer hybrids.1 By combining the sensitizer, which has strong absorption in the visible spectrum, and the radical initiation benzoyl peroxide in the same molecule, we effectively increase the quantum yield of sensitization and thus more efficiently initiate polymerization. These observations highlight the importance of molecular design in identifying highly efficient polymerization initiators.
References
- Greene, F. D.; Kazan, J. Preparation of Diacyl Peroxides with N,N'-Dicyclohexylcarbodiimide. J Org Chem. 28, 2168-2171 (1963).
Skip to...
Videos from this collection:

Now Playing
Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
16.7K Views

Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.6K Views

Glovebox and Impurity Sensors
Inorganic Chemistry
18.6K Views

Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.6K Views

The Evans Method
Inorganic Chemistry
68.4K Views

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
104.4K Views

Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.4K Views

Mössbauer Spectroscopy
Inorganic Chemistry
22.0K Views

Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
38.9K Views

Structure Of Ferrocene
Inorganic Chemistry
79.4K Views

Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
45.2K Views

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.3K Views

Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.3K Views

Dye-sensitized Solar Cells
Inorganic Chemistry
15.8K Views

Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.6K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved