A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Heterotopic and Orthotopic Tracheal Transplantation in Mice used as Models to Study the Development of Obliterative Airway Disease
In This Article
Summary
This video shows and compares two experimental models to study the development of obliterative airway disease (OAD) in mice, the heterotopic and orthotopic tracheal transplantation model.
Abstract
Obliterative airway disease (OAD) is the major complication after lung transplantations that limits long term survival (1-7).
To study the pathophysiology, treatment and prevention of OAD, different animal models of tracheal transplantation in rodents have been developed (1-7). Here, we use two established models of trachea transplantation, the heterotopic and orthotopic model and demonstrate their advantages and limitations.
For the heterotopic model, the donor trachea is wrapped into the greater omentum of the recipient, whereas the donor trachea is anastomosed by end-to-end anastomosis in the orthotopic model.
In both models, the development of obliterative lesions histological similar to clinical OAD has been demonstrated (1-7).
This video shows how to perform both, the heterotopic as well as the orthotopic tracheal transplantation technique in mice, and compares the time course of OAD development in both models using histology.
Protocol
- Male Balb/C mice (8-12 weeks) are purchased from Charles River Laboratories (Sulzfeld, Germany). The mice are housed under conventional conditions, fed standard mice food and water ad libitum.
- 2% Isofluran is used for anesthesia.
DONOR PREPARATION
- Shave the abdominal hair and disinfect the area using betaisodona.
- Under microscopic view, perform a midline cervical incision from the level of the larynx to the sternum.
- Remove the subcutaneous fat and the strap muscles to get a clear view of the trachea.
- Dissect the trachea from any surrounding tissues, like the esophagus, nerves, arteries, and connective tissue.
- Remove the whole trachea (from the larynx to the bifurcation).
- Flush the transplant with cold saline and store the graft at 4°C.
- The donor is euthanized by cervical dislocation following the harvest of the trachea.
RECIPIENT: HETEROTOPIC TRANSPLANTATION
- Shave the abdominal hair in a wide margin around the incision site and disinfect the area three times using betaisodona (betadine) followed by alcohol. Eyes should be lubricated with an ophthalmic ointment product to prevent the corneas from drying out.
- Perform a median laparotomy and place the intestine into a sterile, moistured glove.
- Spread the greater omentum carefully. Place the graft into the center and fixate it with a single suture using 8-0 (Prolene, Ethicon, Germany).
- Fully cover the transplant with the greater omentum and fix the graft with one single suture 8-0 (Prolene, Ethicon, Germany).
- Relocate the intestines back into the abdomen and flush with warm, sterile saline prior to closure.
- Close up in 2 layers - abdominal wall and skin layer with continuous pattern using 7-0 Prolene for the muscle and 7-0 Vicryl for the skin.
RECIPIENT: ORTHOTOPIC TRANSPLANTATION
- Shave the abdominal hair in a wide margin around the incision site and disinfect the area three times using betaisodona (betadine) followed by alcohol. Eyes should be lubricated with an ophthalmic ointment product to prevent the corneas from drying out.
- Divide the strap muscles to visualize the entire laryngotracheal complex.
- Carefully dissect the trachea from the surrounding tissues, take care to preserve the recurrent laryngeal nerves.
- Divide the trachea three rings caudal from the cricoid. The animal maintains physiologic respiration via the tracheostomy.
- Ensure clean tracheal edges in the recipient as well as in the graft.
- The graft is interposed between the recipient tracheal defects and orientated to maintain anatomic polarity.
- Using 8-0 (Prolene, Ethicon, Germany) anastomose the donor graft with the distal (mediastinal) trachea. The posterior aspect of the anastomosis is performed in continuous running fashion. The anterior aspect is then completed using interrupted stitches.
- Remove any secretions from the airway.
- The proximal anastomosis is then completed in the same way as the distal one.
- Ensure integrity of the airway and adequate, spontaneous breathing.
- Relocate the strap muscles and close the subcutaneous tissue and skin layer using 6-0 sutures (Vicryl, Ethicon, Germany) with continuous pattern.
- Use injection anesthesia for the recipient, therefore the animal will retain physiological respiration through the trachea. A combination of 75/1/0.2 mg/kg of propofol, medetomidine and fentanyl, respectively, is used for i.p. anaesthesia in mice.
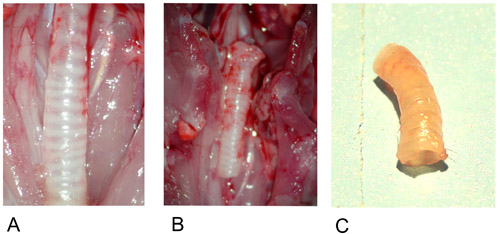
Figure 1: Donor trachea.
1A: Donor trachea in situ after preparation.
1B: Excised donor trachea.
1C: Donor trachea after explantation.
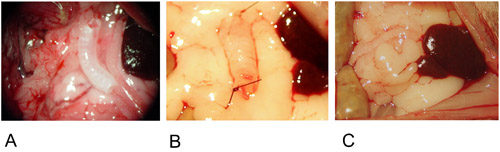
Figure 2: Heterotopic Model.
2A: The graft is positioned in the center of the greater omentum.
2B: The graft is fixed on both ends with a single suture.
2C: The graft is wrapped into the greater omentum and fixed with a single suture.
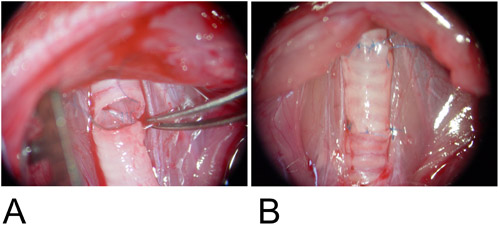
Figure 3: Orthotopic Model.
3A: The graft is interposed between the recipient tracheal defects and the posterior wall is anastomosed in continuous running fashion.
3B: The anterior wall is completed using a single suture.
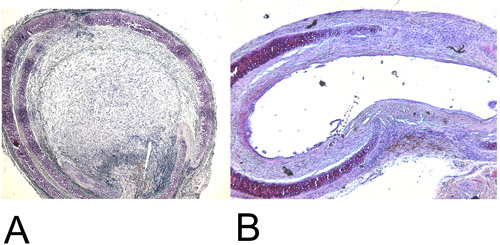
Figure 4: Histology.
4A: Recovered heterotopic transplanted trachea after 28 days in the H+E staining (15x). Note the 100% luminal obliteration.
4B: Recovered orthotopic transplanted trachea after 60 days in the H+E staining (15x). Maximal achieved luminal obliteration is approximately 45%.
| Heterotopic Tracheal Transplantation Model | Orthotopic Tracheal Transplantation Model | |
| Advantages | + Easy to perform + Luminal obliteration with complete airway occlusion after 28 days + No physical affection of animals by OAD |
+ Physical ventilation of the graft + Inhaled drug administration possible + Strong immunological reactions such as alloreactive IgM antibody production + Physiological thoracic milieu + Tracheal-tracheal anastomosis imitates the clinical setting |
| Disadvantages | - No ventilation of transplanted trachea - No evaluation of inhaled pathogens possible - Inhibition of mucociliary clearance and retained secretions - Peritoneal microenvironment instead of thoracic milieu |
- Surgical training necessary - Luminal obliteration with luminal occlusion app. 45% after 60 days - Animals may develop symptoms of OAD |
Table 1: Advantages and Disadvantages of Heterotopic and Orthotopic Tracheal Transplantation.
Discussion
Mice are available in different transgeneic and knockout model, and therefore suitable to study mechanistic questions related to OAD (4).
Both models of tracheal transplantation shown in this video can be used as reliable models for studying OAD development.
However, each model demonstrates advantages and limitations.
The heterotopic tracheal transplantation is easy to perform and does not require special surgical training (3, 5). After het...
Disclosures
Sonja Schrepfer has received a research grant from the Deutsche Forschungsgemeinschaft (DFG) (SCHR992/3–1).]
All animals received human care in compliance with the Principles of Laboratory Animal Care, experiments on animals were performed in accordance with the guidelines and regulations set forth by the National Society for Medical research and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (National Institutes of Health publication 85-23, revised 1985).
All animals were obtained from Charles River Laboratories (Sulzfeld, Germany) and were maintained in the animal care facilities of the University Hospital Hamburg Eppendorf. The animals received standard chow and water ad libitum.
Acknowledgements
The authors thank Christiane Pahrmann (Lab Manager).
References
- Adams, B., Berry, G., Huang, X., Shorthouse, R., Brazelton, T., Morris, R. Immunosuppressive therapies for the prevention and treatment of obliterative airway disease in heterotopic rat trachea allografts. Transplantation. 69, 2260-2266 .
- Adams, B., Brazelton, T., Berry, G., Morris, R. The role of respiratory epithelium in a rat model of obliterative airway disease. Transplantation. 69, 661-665 .
- Deuse, T., Schrepfer, S., Reichenspurner, H., Hoyt, G., Fischbein, M., Robbins, R., Pelletier, M. Techniques for experimental heterotopic and orthotopic tracheal transplantations – when to use which model; Transplant Immunology 17. , 255-261 (2007).
- Hele, D., Yacoub, M., Belvisi, M. The heterotopic tracheal allograft as an animal model of obliterative bronchiolitis. Respiratory Research. 2, 169-183 .
- Hertz, M., Jessurun, J., King, M., Savic, S., Murray, J. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. American Journal of Pathology. 142, (1993).
- McDyer, J. Human and murine obliterative bronchiolitis in transplant. Proceedings of the American Thoracic Society. 4, 37-43 .
- Schrepfer, S., Deuse, T., Hoyt, G., Sheikh, A., Hoffmann, J., Reichenspurner, H., Robbins, R., Pelletier, M. Experimental orthotopic tracheal transplantation: the stanford technique. Microsurgery. 27, 187-189 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved