A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Osmotic Avoidance in Caenorhabditis elegans: Synaptic Function of Two Genes, Orthologues of Human NRXN1 and NLGN1, as Candidates for Autism
In This Article
Summary
Neurexins and neuroligins are membrane-neuron adhesion proteins which perform essential roles in synaptic differentiation and transmission. Neuroligin deficient mutants of C. elegans are defective in detecting osmotic strength, but when they also contain a mutation in the gene coding neurexin, they recover the wild type phenotype.
Abstract
Neurexins and neuroligins are cell adhesion molecules present in excitatory and inhibitory synapses, and they are required for correct neuron network function1. These proteins are found at the presynaptic and postsynaptic membranes 2. Studies in mice indicate that neurexins and neurologins have an essential role in synaptic transmission 1. Recent reports have shown that altered neuronal connections during the development of the human nervous system could constitute the basis of the etiology of numerous cases of autism spectrum disorders 3.
Caenorhabditis elegans could be used as an experimental tool to facilitate the study of the functioning of synaptic components, because of its simplicity for laboratory experimentation, and given that its nervous system and synaptic wiring has been fully characterized. In C. elegans nrx-1 and nlg-1 genes are orthologous to human NRXN1 and NLGN1 genes which encode alpha-neurexin-1 and neuroligin-1 proteins, respectively. In humans and nematodes, the organization of neurexins and neuroligins is similar in respect to functional domains.
The head of the nematode contains the amphid, a sensory organ of the nematode, which mediates responses to different stimuli, including osmotic strength. The amphid is made of 12 sensory bipolar neurons with ciliated dendrites and one presynaptic terminal axon 4. Two of these neurons, named ASHR and ASHL are particularly important in osmotic sensory function, detecting water-soluble repellents with high osmotic strength 5. The dendrites of these two neurons lengthen to the tip of the mouth and the axons extend to the nerve ring, where they make synaptic connections with other neurons determining the behavioral response 6.
To evaluate the implications of neurexin and neuroligin in high osmotic strength avoidance, we show the different response of C. elegans mutants defective in nrx-1 and nlg-1 genes, using a method based on a 4M fructose ring 7. The behavioral phenotypes were confirmed using specific RNAi clones 8. In C. elegans, the dsRNA required to trigger RNAi can be administered by feeding 9. The delivery of dsRNA through food induces the RNAi interference of the gene of interest thus allowing the identification of genetic components and network pathways.
Protocol
1: Osmotic avoidance assay.
- About 16-24 hours before the assay, pick L4 larval stage animals of each genotype to a fresh NGM plate containing OP50 E. coli andincubate it at 20 °C. Next day start the experiment with young adults.
- It is recommended to carry out the assay "blindly". The plates with each strain to be assayed should be relabelled by a second experimenter, performing the assay using the relabelled plates that would be unmasked when the experiment has finished.
- The day of the assay, prepare a 4M stock solution of fructose with 1% Congo Red solution and dissolve completely at room temperature. We recommend checking the solution prior to each assay since the dye could precipitate with time.
- Annular ring (1 cm diameter) on NGM plate is outlined on the centre of the solid medium, with 15 μl of the red 4M fructose solution. Let the fructose solution soak into the agar, this usually takes between 2 and 5 minutes.
- Place individual young adult animals of each strain within the ring and follow over the next 10 minutes to determine the response to the osmotic barrier. Animals avoiding the ring more than six times in a row are classified as normal; those exiting the ring in less than six attempts are considered defective in osmotic sensitivity.
Note: The control strain must be used in each assay. N2 young adult animals are used as positive controls as they decisively avoid the ring barrier. Animals that have been previously starved, passed through Dauer larvae or are coming from plates that are too dry should not be used. At the beginning, control animals should be evaluated in duplicate, to confirm that the assay plates and the solution are correct.
2: Generation of knockdown worms by RNAi feeding.
- RNAi plates: NGM RNAi feeding plates contains per litre: 17 g agar, 2,5 g peptone, 3.0 g NaCl, 1 ml of 5 mg ml-1 cholesterol; fill the flask to 1 litre with H2O and autoclave. After agar cools to about 65 °C, add 25 ml of 1 M KPO4, pH 6.0, 1 ml of 1 M CaCl2, 1 ml of 1M MgSO4, 0,5 ml carbenicillin (50 mg ml-1) and 1 ml 1M IPTG. If you are starting with prepared solid NGM, melt solid medium by microwaving and subsequently place liquid NGM on the bench to cool and then add KPO4, pH 6.0, CaCl2, MgSO4, carbenicillin and IPTG as indicated previously.
Add 10 ml of NGM into each 60 mm Petri dish. Allow cooling and invert plates maintaining them at RT overnight before use. Plates can be stored in a plastic bag at 4 °C for about 4-5 days. - Bacterial preparation and induction: Isolate colonies of E.coli HT115 (DE3) strain, transformed with L4440 vector containing a fragment corresponding to the target gene, onto Luria-Bertani (LB) agar plates (17 g agar, 10 g tryptone, 5 g yeast extract, and 10 g NaCl per litre) containing ampicillin (50 μg/ml) and tetracyclin (15 μg/ml).
Pick a colony of bacteria and inoculate into LB with 50 μg/ml ampicillin, and grown for 6 8 h with shaking at 37 °C; seed a drop of this culture onto the prepared NGM plates and dry the plates thoroughly before being incubating overnight (12 24 h) at room temperature to allow the bacteria to grow and to begin induction. The incubation was in the dark because tetracycline is light sensitive and it may affect the variability of RNAi assays among plates.
It is necessary to use a positive and a negative control for RNAi feeding experiments. The positive control is E.coli HT115 cell transformed with the L4440 vector containing the unc-22 gene sequence. The knockdown of unc-22 gene produces a "twitching phenotype". The negative control is E.coli HT115 cell transformed with empty L4440 vector. - Worm handling and scoring: The first day, place L4 stage worms from an NGM plate seeded with OP50 onto NGM plates without bacteria and incubate at 20 °C for 12 hours (fasting).
The next day, transfer young fasted adult hermaphrodites onto a plate seedded with the bacteria expressing the specific target gene RNAi. Leave 40-48 hours at 20 °C to generate F1 progeny.
Then place a few F1 adult worms onto another plate seeded with the same bacteria. After 24-48 hours, select and isolate from the F2 progeny, young adults (fully formed protruding vulva with few eggs) and score for phenotypes.
Note: the RNAi induction in neurons have some limitations because of the refractory properties of the C. elegans nervous system to RNAi. To overcome the problems of this inefficiency in neuron it is recommended to use the rrf-3 strain, a hypersensitive background to the effects of RNAi in neurons 10. In our experiments no differences were found between Bristol N2 and rrf-3 strains for the genes and the phenotype analyzed.
3: Strains used.
The C. elegans strains used in this work are shown in Table 1. Mutant strains were out-crossed against N2 wild-type at least four times to remove unwanted random mutations that could have been generated during the mutagenesis protocol.
OP50 E. coli strain was suppliied by Caenorhabditis Genetic Center, University of Minnesota, USA. E.coli HT115 (DE3) with the plasmid pL4440 carrying nrx-1 (JA:C29A12.5) and nlg-1 (JA:C40C9.5) gene fragments were provided by Dr. Peter Askjaer, Centro Andaluz de Biología del Desarrollo (CABD), CSIC Universidad Pablo Olavide, Sevilla, Spain.
4: Representative results.
Illustrative results are represented in Figures 1 and 2. Ten animals of each strain and a minimum of three replica experiments were carried out.
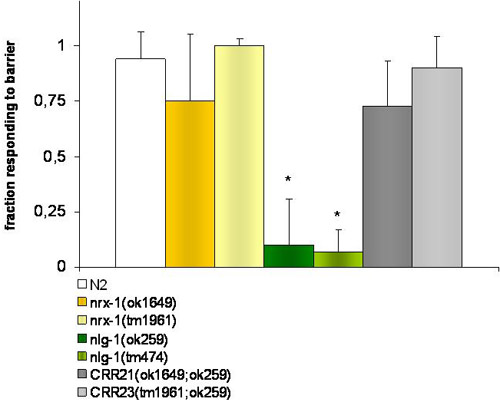
Figure 1. Experiments of osmotic avoidance behavior.
Control strains and nrx-1 (ok1649 and tm1961)V deficient mutantworms respond by reversing backwards when they encounter an osmotic barrier (4M fructose). nlg-1 (ok259 and tm474) X mutants fail to detect this barrier. Double mutants deficient in nlg-1 and nrx-1, strains CRR21 (ok1649 ; ok259)VX and CRR23 (tm1961; ok259)VX recovered the wild type phenotype. The * indicates significant differences (P ≤ 0.001) by t-student test, in the response of each strain to the Bristol N2 wild-type by t-student test
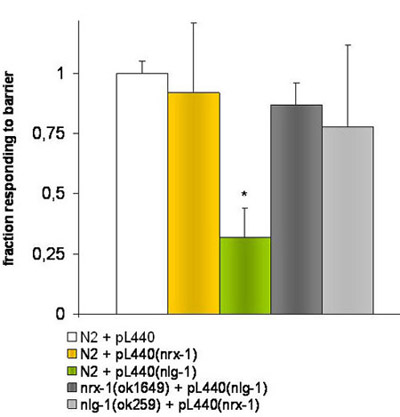
Figure 2. Experiments with knockdown worms by RNAi feeding.
E.coli HT115 (DE3) transformed with empty pL4440 vector or containing a fragment corresponding to the target genes nrx-1 or nlg-1 were used to fed different worm strains. The * indicates significant differences (P ≤ 0.001) by t-student test, in the response of the N2 strain fed with bacteria carrying the pL4440 vector with a fragment targeting the nlg-1 gene in comparison with the N2 strain fed with empty pL4440 vector.
Discussion
Neurexins and neuroligins perform essential roles in synaptic transmission11 and differentiation of synaptic connections 12. Both molecules have been identified as candidate genes for autism 13,14.
In this video we show a simple method which allows us to study the effect of genes affecting the osmotic avoidance response in C. elegans. Neuroligin deficient mutants are defective in detecting osmotic strength; but mutants deficient in neurexin show a res...
Acknowledgements
We would like to thanks Dr Antonio Miranda-Vizuete for his valuable help. We also like to express our gratitude to Salma Boulayoune and Isabel Caballero for valuable technical assistance. This work was financed by a grant from the Junta de Andalucıa (BIO-272). This research has been carried out in agreement with current laws governing genetic experimentation in Europe.
Materials
| Name | Company | Catalog Number | Comments | ||||||||||||||||||||||||||||||||||||
Table 1. C. elegans strains.
a. Caenorhabditis Genetic Center, University of Minnesota, USA. | |||||||||||||||||||||||||||||||||||||||
References
- Sudhof, T. C. . Nature. 455 (7215), 903-903 (2008).
- Fabrichny, I. P., Leone, P., Sulzenbacher, G. . Neuron. 56 (6), 979-979 (2007).
- Garber, K. . Science. 317 (5835), 190-190 (2007).
- Wang, K., Zhang, H., Ma, D. . Nature. 459 (7246), 528-528 (2009).
- Ward, S., Thomson, N., White, J. G. . The Journal of comparative neurology. 160 (3), 313-313 (1975).
- Bargmann, C. I., Thomas, J. H., Horvitz, H. R. Cold Spring Harbor symposia on quantitative biology. 55, 529-529 (1990).
- White, J. G., Southgate, E., Thomsom, J. N., Brenner, S. . Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1-1 (1986).
- Culotti, J. G., Russell, R. L. . Genetics. 90 (2), 243-243 (1978).
- Fire, A., Xu, S., Montgomery, M. K. . Nature. 391 (6669), 806-806 (1998).
- Timmons, L., Fire, A. . Nature. 395 (6705), 854-854 (1998).
- Simmer, F., Tijsterman, M., Parrish, S., Koushika, S. P., Nonet, M. L., Fire, A., Ahringer, J., Plasterk, R. H. . Curr Biol. 12, 1317-1317 (2002).
- Missler, M., Zhang, W., Rohlmann, A. . Nature. 423 (6943), 939-939 (2003).
- Varoqueaux, F., Aramuni, G., Rawson, R. L. . Neuron. 51 (6), 741-741 (2006).
- Graf, E. R., Zhang, X., Jin, S. X., Scheiffele, P., Fan, J., Choih, J. . Cell. 119 (7), 1013-1013 (2004).
- Scheiffele, P., Fan, J., Choih, J. . Cell. 101 (6), 657-657 (2000).
- Jamain, S., Quach, H., Betancur, C. . Nature genetics. 34 (1), 27-27 (2003).
- Szatmari, P., Paterson, A. D., Zwaigenbaum , L. . Nature genetics. 39 (3), 319-319 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved