A subscription to JoVE is required to view this content. Sign in or start your free trial.
Spinal Cord Neuron Isolation: A Density Gradient Procedure to Isolate Neonatal Spinal Cord Neurons for In Vitro Studies
Summary
[INSERT FIGURE1 HERE]
Figure 1: Spinal column dissected and spinal cord released from a 3 day-old neonatal mouse. The arrow in (a) points towards the caudal end of the spine, where a needle is inserted to release the spinal cord. A released spinal cord (b) is shown submerged in PBS.
[INSERT FIGURE2 HERE]
Figure 2: Density gradient, with cells added-on after centrifugation. The layers are outlined and better visualized on the cartoon depiction. The most superficial layer (0) is generally debris from the spinal cord tissue. Layer 1 is rich in oligodendrocytes and astrocytes. Layers 2 and 3 contain the majority of neurons. While layer 3 contains neurons with the highest purity, some supporting cells (i.e., astrocytes and oligodendrocytes) are found in layer 2. The pellet at the bottom mainly contains microglial cells.
Overview
In this video we describe a step-by-step protocol to isolate spinal cord neurons from a neonatal mouse for in vitro studies.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Harvesting Spinal Cords
NOTE: All instruments should be autoclaved (135 °C and 30 psi for 4 x 7 min cycles) for sterility.
- Euthanize 1–3 day-old C57BL/6 mouse pups in a chamber with isoflurane. Wait 30 s after the cessation of movement and pinch the leg to confirm a lack of response.
- Separate the head from the body using scissors, with pup in the prone position.
- Stabilize the hind legs or tai....
Representative Results
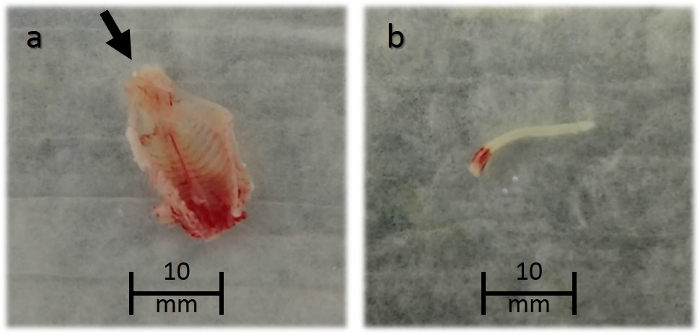
Figure 1: Spinal column dissected and spinal cord released from a 3 day-old neonatal mouse. The arrow in (a) points towards the caudal end of the spine, where a needle is inserted to release the spinal cord. A released spinal cord (b) is shown submerged in PBS.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Source: Eldeiry, M. et al. Spinal Cord Neurons Isolation and Culture from Neonatal Mice. J. Vis. Exp. (2017)
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved