A subscription to JoVE is required to view this content. Sign in or start your free trial.
Differentiating Embryoid Body Cells into Neural Progenitors Using Retinoic Acid
In This Article
Overview
The video demonstrates the method to induce stem cell differentiation into neural progenitors using retinoic acid and the hanging drop culture method.
Protocol
1. Culture of Mouse Embryonic Fibroblasts (MEFs)
- Prepare MEF medium, Dulbecco's modified Eagle's medium (DMEM, high-glucose), supplemented with 15% fetal bovine serum (FBS).
- Coat 100 mm cell culture dishes with 0.5% gelatin solution for 30 min at room temperature (RT).
- Count MEFs using a cytometer. Remove the gelatin solution and immediately pour MEF medium pre-warmed to 37 °C. Rapidly thaw vials of mitomycin C-treated MEFs in a 37 °C water bath for 2 min, then seed 2.8 x 106 MEFs per 100 mm gelatin-coated dish. Adjust cell number accordingly if using dishes of other sizes. Incubate MEFs overnight at 37 °C, 5% CO2.
- Change the medium on the next day. Culture for 2-3 days until the MEF layer is confluent.
2. Mouse Embryonic stem cell or ESC Culture
- Prepare ESC medium, Iscove's modified Dulbecco's medium (IMDM) supplemented with 15% FBS and 103 U/mL leukemia inhibitory factor (LIF), 0.1 mM nonessential amino acids, 55 mM 2-mercaptoethanol, penicillin (100 U/mL), streptomycin (100 µg/mL), gentamicin (200 µg/mL), and 0.2% mycoplasma antibiotic.
- Remove MEF medium from the dish prepared in step 1 and replace it with 37 °C pre-warmed ESC medium.
- Defrost an ESC vial and seed cells on top of the MEF layer. Incubate at 37 °C, 5% CO2, until ESCs reach confluence.
- Prepare new cell culture dishes containing a confluent monolayer of MEFs. To passage the ESCs, wash once with PBS and detach with 0.25% trypsin/EDTA for 2-5 min at 37 °C, 5% CO2. Stop the trypsinization by adding fresh IMDM with 15% FBS to the detaching cells, transfer the cell suspension to a 15 mL tube, and centrifuge at 160 x g for 5 min at RT.
- Remove the supernatant, resuspend ESCs with fresh IMDM, and passage one-fifth of the cells to each new MEF-coated dish; incubate until cells reach confluence (typically 4-5 days).
3. Withdraw MEFs and Culture ESCs on Gelatin-coated Plates
- Once ECSs reach confluence, detach them and MEFs with 0.25% Trypsin/EDTA as in step 2.4, resuspend the cells in fresh IMDM, transfer to a non-adhesive bacteriological petri dish, and incubate for 40 min at 37 °C, 5% CO2.
- Carefully transfer ESCs and MEFs-containing medium to a new gelatin-coated plate using a 5 mL pipette, avoiding repeated pipetting. The cells remaining in the petri dishes are MEFs since ESCs do not adhere.
- Passage the ESCs every 3-4 days. Less frequent passaging may reduce ESC pluripotency. Do not exceed 60% confluence, as it may favor differentiation.
- Repeat steps 3.2-3.3 three times or more, as required, until MEFs are no longer detectable by a cell culture microscope, using a 20X objective, to make sure MEFs are not present.
- Verify ESC pluripotency by checking ESC culture to see if the cells form dense colonies with typical ESC polygonal morphology (Figure 1A). Test cell stemness by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), immunoblotting, or immunofluorescence of core pluripotency transcription factors (Figure 2).
4. EB Formation
- Prepare an all-trans-RA stock solution at 10 mM in DMSO and aliquot it into 1.5 mL light-protected microfuge tubes. The solution is stable at -80 °C for up to two weeks. Protect it from light.
- Trypsinize ESCs as in step 2.4; replate in fresh IMDM without LIF as a single-cell suspension.
- Count cells using a hemocytometer, and prepare a 5 x 105 cells/mL suspension in IMDM with 0.5 µM RA.
- Plate 100 20-µL-drops per 100-mm petri dish with an 8-channel pipette and 200 µL tips, invert the dishes, and fill the inverted lid with PBS to prevent hanging drops from drying. Protect RA-containing culture media from light.
- Culture EBs in hanging drops at 37 °C, 5% CO2, for 4 days.
Results
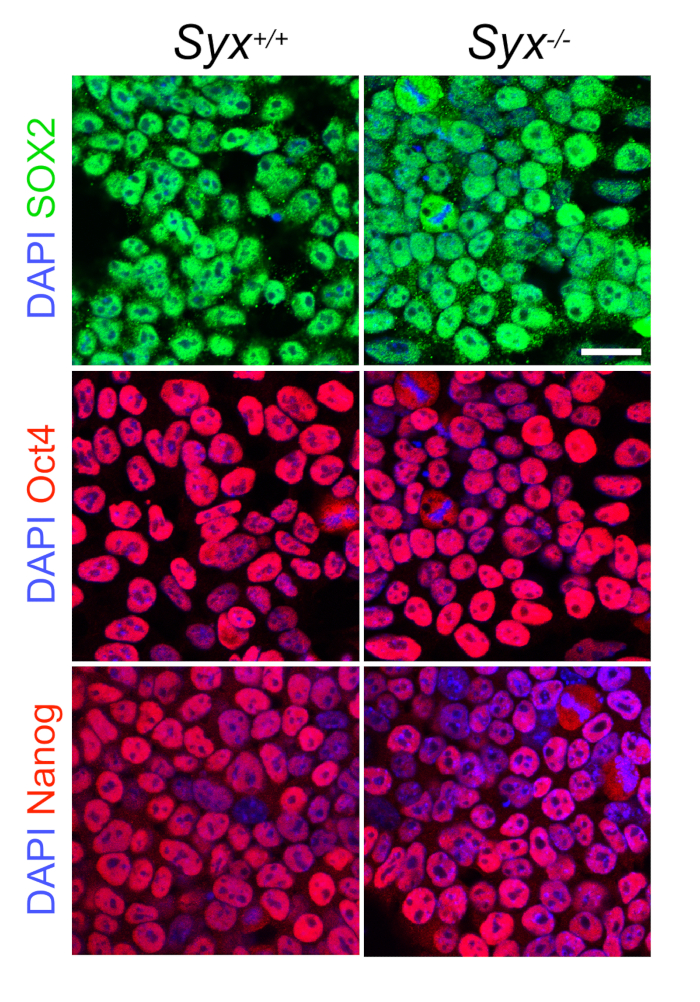
Figure 1: Sprout Emergence from Syx+/+ and Syx-/- EBS in 3D Culture. (A) Syx+/+ and Syx-/- ESCs grew in clustered colonies before the induction of neural differentiation. (B and C) images of EBs formed in hanging drops with 0.5 µM RA, and then inserted into a 3D collagen matrix without RA. Images were captured on day 6 by the indicated objectives (...
Disclosures
Materials
| Name | Company | Catalog Number | Comments |
| Materials | |||
| MEFs | EMD Millipore | PMEF-CF | ESC feeder layer |
| ESC | EMD Millipore | CMTI-2 | |
| Cell culture dish (60 mm) | Eppendorf | 30701119 | Cell culture |
| Cell culture dish (100 mm) | Falcon | 353003 | Cell culture |
| Petri dish (100 mm) | Corning | 351029 | Hanging drops |
| Dark 1.5 ml centrifuge tube | Celltreat Scientific Products | 229437 | RA stock solution |
| Microscope cover-glass | Fisherbrand | 12-545-80 | Circular, 12 mm diameter |
| Superfrost-plus microscope slides | Fisherbrand | 12-550-15 | |
| Reagents | |||
| DMEM | Lonza | 12-709F | MEFs culture |
| IMDM | Gibco | 12440-046 | ESCs culture |
| Fetal bovine serum (FBS) | EMD Millipore | ES-009-B | ESCs culture |
| Gelatin | Sigma-Aldrich | G2625 | Dish coating |
| LIF | R&D Systems | 8878-LF-025 | To maintain ESC pluripotency |
| MEM Non-Essential Amino Acids Solutions | Gibco | 11140050 | Cell culture |
| 2-Mercaptoethanol | Gibco | 21985023 | Cell culture |
| Penicillin-Streptomycin | Gibco | 15140122 | Cell culture |
| Gentamicin | Gibco | 15750060 | Cell culture |
| MycoZap Plus-PR | Lonza | VZA-2022 | Cell culture |
| 0.25% Trypsin-EDTA | Gibco | 25200-072 | Cell culture |
| DMSO | Sigma-Aldrich | D2650 | |
| All-trans-retinoic acid | Sigma-Aldrich | R2625-50MG | Induction of neural differentiation |
| PBS | Gibco | 10010049 | |
| Instruments | |||
| Wide-field microscope | Nikon | Eclipse TS100 | Cell culture imaging |
This article has been published
Video Coming Soon
Source: Yang, J., et al. Analysis of Retinoic Acid-induced Neural Differentiation of Mouse Embryonic Stem Cells in Two and Three-dimensional Embryoid Bodies. J. Vis. Exp. (2017).
Copyright © 2025 MyJoVE Corporation. All rights reserved