A subscription to JoVE is required to view this content. Sign in or start your free trial.
Simultaneous Measurement of Intracellular Calcium and Membrane Potential in a Mouse Cerebral Endothelium
In This Article
Overview
The video demonstrates the simultaneous measurement of intracellular calcium levels and changes in membrane potential in endothelial cells of mouse cerebral artery segments. The isolated endothelial tube is loaded with a fluorescent calcium indicator and treated with a drug that induces intracellular calcium release, resulting in a change in membrane potential. The indicator's fluorescence enables the measurement of calcium levels, and a recording electrode is inserted into a cell to measure changes in membrane potential.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Dissection and Isolation of Cerebral Artery
NOTE: All dissection procedures require specimen magnification (up to 50x) via stereomicroscopes and illumination provided by fiber optic light sources. To perform dissection procedures for isolation of the brain and arteries, use sharpened dissection instruments. Microdissection tools to isolate and clean arteries include sharpened fine-tipped forceps and Vannas style dissection scissors (3 to 9.5 mm blades).
- Isolation of Mouse Brain
- Anesthetize a C57BL/6 mouse (3-30 months old; male or female) via inhalation of isoflurane (3% for 2–3 min), then decapitate the animal immediately following complete induction of anesthesia. Place the head in a Petri dish (diameter 10 cm, depth 1.5 cm) containing cold (4 °C) zero Ca2+ Physiological salt solution (PSS) under a stereomicroscope.
- Viewing through the microscope, remove the skin and hair over the skull and remove excessive blood with cold zero Ca2+ PSS. Make an incision using only the tips of standard dissection scissors (e.g., 24 mm blade), starting with the occipital bone and extending up through the nasal bone of the skull.
- Open the skull carefully along the incision using coarse-tipped forceps, and separate connective tissue to isolate the brain with an intact Circle of Willis.
NOTE: Alternatively, Littauer bone-cutting forceps can be used to open the skull and expose the brain. - Gently wash the isolated brain with cold zero Ca2+ PSS in a beaker to remove blood. Place the brain ventral side facing up in a chamber containing cold dissection solution for isolation of cerebral arteries (Figure 1A).
- Isolation of Cerebral Arteries
NOTE: The posterior cerebral artery has been selected as a representative cerebrovascular endothelial study model for this protocol.- Secure the isolated brain in cold dissection solution using stainless steel pins (diameter 0.2 mm, length ~11–12 mm) to be inserted into a charcoal-infused silicon polymer coating the bottom (depth ≥50 cm) of a glass Petri dish.
- To maintain a chilled temperature of PSS during dissection, use a dissection chamber cooled by a refrigerant system continuously cycling a 1:1 water-ethylene glycol mixture. Alternatively, dissection can be performed on fresh ice.
- Surgically isolate the posterior cerebral arteries (~0.3 to 0.5 cm segments, no axial tension) from the posterior communicating and basilar arteries using microdissection instruments Vannas style scissors and sharpened fine-tipped forceps (Figure 1B). Use stainless steel pins (diameter 0.1 mm, length ~13–14 mm) to secure both isolated posterior cerebral arteries in the dissection solution in the Petri dish (Figure 1C).
- Clean the isolated posterior cerebral arteries carefully by removing connective tissue using sharpened fine-tipped forceps (Figure 1D). Cut intact arteries into segments (length 1–2 mm) for enzymatic digestion (Figure 1D; inset).
2. Preparation of Endothelial Tube and Superfusion
- Prepare the trituration apparatus using a microscope equipped with objectives (10x, 20x, and 40x), a camera, and an aluminum stage holding a chamber and micromanipulators. Secure a microsyringe with a pump controller adjacent to the stage and specimen (Figure 2A).
- Completely backfill a trituration pipette with mineral oil and secure it over the micro-syringe piston. Then, using the micro-syringe with pump controller, withdraw dissociation solution into the pipette (~130 nl) on top of the mineral oil while ensuring the absence of air bubbles in the pipette.
- Place intact arterial segments into 1 mL of dissociation solution in a 10 mL glass tube (Figure 2B), containing 0.31 mg/mL papain, 0.5 mg/mL dithioerythritol, 0.75 mg/mL collagenase, and 0.13 mg/mL elastase. Incubate at 34 °C for 10–12 min for partial digestion.
- Following the digestion, replace the enzyme solution with ~5 mL of fresh dissociation solution. Using a 1 mL pipette, transfer one segment into a chamber containing the dissociation solution at room temperature (RT).
- Place the pipette into the dissociation solution in the chamber and position it close to one end of the digested vessel. Set a rate within the range of 2 to 5 nL/s on the pump controller for gentle trituration (Figure 2C; inset).
- While viewing through 100x to 200x magnification, withdraw and eject the arterial segment to dissociate smooth muscle cells while producing an endothelial tube. If necessary, carefully use fine-tipped forceps to separate dissociated adventitia and internal elastic lamina from the endothelial tube. Confirm that all smooth muscle cells are dissociated and that only endothelial cells remain as an intact "tube" (Figure 2C).
- Using micromanipulators, secure each end of the endothelial tube on the glass cover slip of the superfusion chamber using borosilicate glass pinning pipettes (Figure 2D).
- Wash-out dissociated adventitia and smooth muscle cells from the chamber and replace the dissociation solution with 2 mM CaCl2 PSS. Transfer the mobile platform with secured endothelial tube onto the microscope of superfusion and experimental rig.
- Use 6 clean 50 mL reservoirs (Figure 3A) for continuous delivery of PSS and respective drug solutions during the experiment, as appropriate. Use the inline flow control valve to manually set the flow rate throughout as consistent with laminar flow while matching flow feed to vacuum suction. Deliver PSS to the chamber (Figure 3B) for the superfusion of the endothelial tube for ≥ 5 min before recording the background data and dye loading.
3. Dye Load, Wash-Out, and Temperature Settings
- Turn on all components of the photometry system for measuring [Ca2+]i. Use the microscope on the experimental rig (Figure 3C) to view the endothelial tube at 400x magnification, and focus on cells in the photometric window using the photometry system software suite.
- Measure the diameter of the endothelial tube and record background autofluorescence values in accord with 510 nm emission during alternate excitation at 340 and 380 nm (≥10 Hz).
- To measure intracellular Ca2+ ([Ca2+]i) responses, load the endothelial tube with Fura-2 AM dye (Fura-2-acetoxymethyl ester, 10 µM final concentration) at RT and allow ~30–40 min in the absence of light.
- Restart superfusion of the endothelial tube with fresh PSS for ~30–40 min to wash-out excess dye and allow intracellular Fura-2 AM to de-esterify. During the wash-out period, raise the temperature gradually from RT to 37 °C using the temperature controller (Figure 3A) with an inline heater, then maintain at 37 °C throughout the experiment.
NOTE: The recommended incremental transition between RT to 37 °C entails three steps (e.g., 5 °C) with a ≥5 min equilibration time at each step.
4. Simultaneous Measurement of [Ca2+]i and Vm
- Turn on all other equipment and the electrometer software suite for measuring membrane potential (Vm, see Figure 3, Figure 4). Adjust data acquisition rate accordingly (≥10 Hz).
- Pull a sharp electrode and backfill with 2 M KCl. For studies of intercellular coupling via dye transfer, backfill the microelectrodes with 0.1% propidium iodide dissolved in 2 M KCl.
- Secure the electrode over a silver wire coated with chloride in the pipette holder attached to an electrometer head stage secured with a micromanipulator. Use the micromanipulator to briefly position the tip of the electrode into the flowing PSS in the chamber while viewing through the 4x objective.
- Set the resting Vm to 0 as consistent with grounded bath potential. Position the tip of electrode just over a cell of the endothelial tube. If desired, use audible baseline monitors linked to electrometers to associate sound pitch with potential recordings.
- Increase magnification to 400x using the 40x objective and re-position the tip of the electrode as needed. Adjust the photometric window using the photometry software to focus on ~50 to 80 endothelial cells.
- Gently place the electrode into one of the cells of the endothelial tube using the micromanipulator and wait ≥2 min for the resting Vm to stabilize. Similarly, insert a second electrode in another cell (distance ≥100 µm) from the first cell impaled with first electrode.
- Once resting Vm is stable at its expected values (-30 to -40 mV), turn on the PMT on the fluorescence interface in the absence of light and begin acquisition of intracellular [Ca2+]i by exciting Fura-2 alternately (10 Hz) at 340 and 380 nm while collecting fluorescence emission at 510 nm.
NOTE: To test intercellular electrical coupling, current (± 0.5-3 nA, ~20 s pulse duration) can be delivered via one electrometer as "Site 1", and changes of Vm can be recorded with another electrometer as "Site 2" (distance ≥100 µm). - Once simultaneous measurements of Vm and [Ca2+]i are established, allow ~5 min for superfusion of endothelial tube with PSS at a constant laminar flow rate before application of drugs.
- Apply the drug (e.g., adenosine triphosphate [ATP]) prepared in PSS to the superfusion chamber with the constant flow rate. After ~3 min (or a period of time appropriate for the kinetics of the drug), wash with PSS until Vm and the F340/F380 ratio return to their baseline conditions. Mark respective recordings as temporally synchronized across the photometer and electrometer software suites during each transition of solutions.
- Once the experiment is done, withdraw electrode from the cell using the micromanipulator and notice Vm ~0 mV as referenced by the bath electrode. Stop respective recordings of Vm and [Ca2+]i and save the records on file for data analysis.
Results
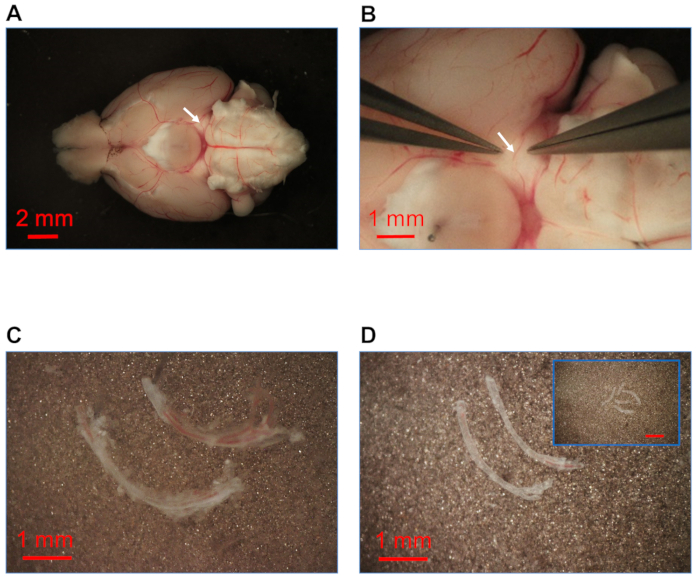
Figure 1: Isolation of cerebral artery from brain. (A) Brain isolated with intact arteries (white arrow indicates the posterior cerebral artery) in dessection solution. (B) Magnified view of posterior cerebral artery (white arrow; ~3x vs. Panel A). (C) Isolated posterior cerebral arteries secured with stainless steel pins in sp...
Disclosures
Materials
| Name | Company | Catalog Number | Comments |
| Glucose | Sigma-Aldrich (St. Louis, MO, USA) | G7021 | |
| NaCl | Sigma | S7653 | |
| MgCl2 | Sigma | M2670 | |
| CaCl2 | Sigma | 223506 | |
| HEPES | Sigma | H4034 | |
| KCl | Sigma | P9541 | |
| NaOH | Sigma | S8045 | |
| ATP | Sigma | A2383 | |
| HCl | ThermoFisher Scientific (Pittsburgh, PA, USA) | A466250 | |
| Collagenase (Type H Blend) | Sigma | C8051 | |
| Dithioerythritol | Sigma | D8255 | |
| Papain | Sigma | P4762 | |
| Elastase | Sigma | E7885 | |
| BSA | Sigma | A7906 | |
| Propidium iodide | Sigma | P4170 | |
| DMSO | Sigma | D8418 | |
| Fura-2 AM dye | Invitrogen, Carlsbad, CA, USA | F14185 | |
| Recirculating chiller (Isotemp 500LCU) | ThermoFisher Scientific | 13874647 | |
| Plexiglas superfusion chamber | Warner Instruments, Camden, CT, USA | RC-27 | |
| Glass coverslip bottom (2.4 × 5.0 cm) | ThermoFisher Scientific | 12-548-5M | |
| Anodized aluminum platform (diameter: 7.8 cm) | Warner Instruments | PM6 or PH6 | |
| Compact aluminum stage | Siskiyou, Grants Pass, OR, USA | 8090P | |
| Micromanipulator | Siskiyou | MX10 | |
| Stereomicroscopes | Zeiss, NY, USA | Stemi 2000 & 2000-C | |
| Fiber optic light sources | Schott, Mainz, Germany & KL200, Zeiss | Fostec 8375 | |
| Nikon inverted microscope | Nikon Instruments Inc, Melville, NY, USA | Ts2 | |
| Phase contrast objectives | Nikon Instruments Inc | (Ph1 DL; 10X & 20X) | |
| Fluorescent objectives | Nikon Instruments Inc | 20X (S-Fluor), and 40X (Plan Fluor) | |
| Nikon inverted microscope | Nikon Instruments Inc | Eclipse TS100 | |
| Microsyringe pump controller (Micro4 ) | World Precision Instruments (WPI), Sarasota, FL, USA | SYS-MICRO4 | |
| Vibration isolation table | Technical Manufacturing, Peabody, MA, USA | Micro-g | |
| Amplifiers | Molecular Devices, Sunnyvale, CA, USA | Axoclamp 2B & Axoclamp 900A | |
| Headstages | Molecular Devices | HS-2A & HS-9A | |
| Function generator | EZ Digital, Seoul, South Korea | FG-8002 | |
| Data Acquision System | Molecular Devices, Sunnyvale, CA, USA | Digidata 1550A | |
| Audible Baseline Monitors | Ampol US LLC, Sarasota, FL, USA | BM-A-TM | |
| Digital Storage Oscilloscope | Tektronix, Beaverton, Oregon, USA | TDS 2024B | |
| Fluorescence System Interface, ARC Lamp + Power Supply, Hyperswitch, PMT | Molecular Devices, Sunnyvale, CA, USA | IonOptix Systems | |
| Temperature Controller | Warner Instruments | TC-344B or C | |
| Inline Heater | Warner Instruments | SH- 27B | |
| Valve Controller | Warner Instruments | VC-6 | |
| Inline Flow Control Valve | Warner Instruments | FR-50 | |
| Electronic Puller | Sutter Instruments, Novato, CA, USA | P-97 or P-1000 | |
| Microforge | Narishige, East Meadow, NY, USA | MF-900 | |
| Borosilicate Glass Tubes (Trituration) | World Precision Instruments (WPI), Sarasota, FL, USA | 1B100-4 | |
| Borosilicate Glass Tubes (Pinning) | Warner Instruments | G150T-6 | |
| Borosilicate Glass Tubes (Sharp Electrodes) | Warner Instruments | GC100F-10 | |
| Syringe Filter (0.22 µm) | ThermoFisher Scientific | 722-2520 | |
| Glass Petri Dish + Charcoal Sylgard | Living Systems Instrumentation, St. Albans City, VT, USA | DD-90-S-BLK | |
| Vannas Style Scissors (3 mm & 9.5 mm) | World Precision Instruments | 555640S, 14364 | |
| Scissors 3 & 7 mm blades | Fine Science Tools (or FST), Foster City, CA, USA | Moria MC52 & 15000-00 | |
| Sharpened fine-tipped forceps | FST | Dumont #5 & Dumont #55 |
This article has been published
Video Coming Soon
Source: Hakim, M. A. et al. Simultaneous Measurements of Intracellular Calcium and Membrane Potential in Freshly Isolated and Intact Mouse Cerebral Endothelium. J. Vis. Exp. (2019)
Copyright © 2025 MyJoVE Corporation. All rights reserved