A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
An Organotypic Slice Assay for High-Resolution Time-Lapse Imaging of Neuronal Migration in the Postnatal Brain
In This Article
Summary
This protocol describes an organotypic slice assay optimized for the postnatal brain and high-resolution time-lapse imaging of neuroblast migration in the rostral migratory stream.
Abstract
Neurogenesis in the postnatal brain depends on maintenance of three biological events: proliferation of progenitor cells, migration of neuroblasts, as well as differentiation and integration of new neurons into existing neural circuits. For postnatal neurogenesis in the olfactory bulbs, these events are segregated within three anatomically distinct domains: proliferation largely occurs in the subependymal zone (SEZ) of the lateral ventricles, migrating neuroblasts traverse through the rostral migratory stream (RMS), and new neurons differentiate and integrate within the olfactory bulbs (OB). The three domains serve as ideal platforms to study the cellular, molecular, and physiological mechanisms that regulate each of the biological events distinctly. This paper describes an organotypic slice assay optimized for postnatal brain tissue, in which the extracellular conditions closely mimic the in vivo environment for migrating neuroblasts. We show that our assay provides for uniform, oriented, and speedy movement of neuroblasts within the RMS. This assay will be highly suitable for the study of cell autonomous and non-autonomous regulation of neuronal migration by utilizing cross-transplantation approaches from mice on different genetic backgrounds.
Protocol
I. Procedures
The following techniques should be performed under sterile conditions, in a laminar flow hood, using sterilized tools.
Preparation of glass bottom dishes for organotypic slices
- Dishes must be prepared in sterile environment and using sterilized tools.
- A 150μL drop of slice medium (see recipes) is placed in the center of the glass-bottom part of the dish with care to avoid air bubbles in the medium.
- With a disposable syringe equipped with a 23 Gauge needle, multiple spots of glue (Rubber Cement - Elmer's, cat# E904) are placed on the square fringes adjacent to the circular coverslip that occupies the center of the culture dish, while leaving one side unglued for fluid exchange (Figure 1). The listed type of glue is not toxic to the slices or the cells as applied in this protocol. Care must be given to avoid placing any glue on the glass cover slip as this will impinge upon and obstruct imaging of the slices later. A nucleopore membrane (diameter 25mm, pore size 8.0μm - Whatman, cat# 110614) is placed on top of the glass coverslip, using the glue spots to secure it in place. This should be done using a micro forceps, while ensuring that air bubbles are not trapped between the glass coverslip and the membrane.
- Add 1 mL slice medium on top of membrane. Dishes are placed in an incubator for 30 minutes and then on ice, until ready to use.
Extraction of early postnatal brains
The best results are obtained when slices are prepared from young postnatal mice (P1-P10).
- Pups are terminally anesthetized (overdosed) by isofluorane or other approved methods. The head can be sprayed with 70% ethanol to increase sterility, followed by rapid decapitation using sharp scissors.
- The head is stabilized by clamping the mandible with micro forceps. The skin is excised longitudinally from the neck to the snout. The skull is cut longitudinally and anteriorly starting at the cisterna magna, by making 1 medial and 2 lateral cuts (one on each side - Figure 2A). Care should be taken to minimize contact with underlying cortical tissue as the cranial flaps are removed away from the brain.
- To improve stability of the tissue during vibratome sectioning, the lateral-most aspects of the brain are removed by making two sagittal cuts. The caudal aspect of the brain is also removed by making a cut at the rostral base of the cerebellum (Figure 2B).
- The two hemispheres are separated by making a smooth cut along the midline fissure, and the two hemispheres are carefully scooped out of the skull and placed, medial surface down, in an embedding mold (Figs. 2C-D).
Sectioning of the host brain
- The two hemispheres in the embedding mold are immediately covered with melted 3% low melting point DNA-grade agarose gel (Fisher, cat# BP1360-100) dissolved in tissue preparation buffer that is maintained at 37°C (see recipes). After 2 minutes of stabilization on a flat horizontal surface to ensure even hardening of the agarose, the molds are positioned on ice to complete the setting.
- Once the gel containing the hemispheres is set, it is removed from the mold and trimmed, leaving 2-3mm of gel around the brain tissue.
- The gel-embedded tissue is then mounted on the specimen disc of the vibratome, with the medial surface up, and secured with cyanoacrylate adhesive (Krazy glue or equivalent). Care must be taken to apply only minimal amounts of glue, as too much will have toxic effects on slices and migrating cells. Too much glue on the sides of the block will also impede the cutting, causing potential damage in the tissue.
- The disc is installed in the vibratome specimen tray filled with ice-cold tissue preparation medium.
- The tissue is sectioned at 150μm thickness, with the vibratome speed set on a slow-to-medium range (will depend on the vibratome used, and thus must be determined independently for optimal results). In our hands, the vibration frequency is optimal when set at maximum. The first few slices may be discarded.
- As soon as the RMS-containing slices are released from the blade (the RMS is visible by the naked eye as a grey U-shaped structure extending from the SEZ to the OB), they are carefully teased out of the gel mold, and scooped using a small flathead spatula. The slices are placed on the nucleopore membrane of the glass-bottom dishes containing slice medium (Figure 3A-B). A typical early postnatal mouse brain will yield approximately 2-3 RMS sections per hemisphere when cut at 150 μm. It is critical that handling the slices is kept to a minimum as they are very fragile. The dishes with the slices are then transferred to an incubator.
Donor brain sectioning and RMS transplantation
- Donor brains (brains expressing fluorescent reporters in migrating neuroblasts) are sectioned at 250μm thickness and slices are collected in ice-cold tissue preparation buffer.
- Slices are immediately placed under a dissecting microscope with epifluorescence capability, and the RMS is gently microdissected, using micro forceps. One forceps is used to stabilize the slice while the other is used to make small cuts around the RMS until it is released from the slice. The excised RMS is then cut into small explants (approximately 200-500 μm in diameter) prior to transplantation.
- Glass-bottom dishes containing host slices positioned on the nucleopore filters are removed from the incubator and placed under the dissecting microscope. Using visible light, the RMS is clearly identified and a small incision is made in the initial segment of the RMS.
- Using a pipettor equipped with a 20 μL tip, a single donor RMS explant is transferred to the incised site in the host RMS. The explant is gently pushed into the incision to establish contact between the 2 tissues. To ensure this contact is stable, the explants are pushed slightly in between the slice and the nucleopore membrane.
- Once all host slices with the RMS are transplanted, the dishes are returned to the incubator for at least 1 hour to allow the sections to settle on the membrane. Neuroblasts should begin to migrate from the explant into the host RMS after approximately 1-2 hours.
Time-lapse imaging of neuronal migration
- The glass-bottom dishes are transferred from the incubator to the incubated chamber on the microscope (Figure 3C). Fluorescent neuroblasts can be imaged at intervals ranging from 0 to 10 minutes depending on the type of analysis desired. For example we image cytoskeletal dynamics during the migratory cycle of individual neuroblasts by setting our intervals at less than or equal to 2.5 minutes. However, population dynamics such as orientation and speed of migration are better captured at intervals ranging from 5 to 10 minutes. The choice of objectives is highly variable depending on the brand of microscope. For a Nikon C1 confocal microscope, we find that the 20x dry lens (Nikon Pan Fluor, N.A. 0.75, W.D. 0.35mm) is most suitable for our analyses. For best results on this confocal system, the pinhole is opened to a medium size (60μm diameter). For most appropriate migratory behavior, imaging is confined to cells deep within the thickness of the RMS, at least 20 μm away from either of the cut surfaces of the slice. Laser power should be optimized so that it is at a minimum, but that the details of individual neuroblasts remain visible.
- Once the imaging is completed slices can be fixed with ice cold, and freshly prepared 4% paraformaldehyde, immunostained, and mounted on slides for further imaging. Since we do not coat our membranes with any adherent substrates such as laminin, the slices usually float away from the membrane once they are immersed in the staining buffers. Sections are imaged while maintaining their 150 μm thickness. It is also possible to cryopreserve and freeze the slices and use a cryostat to obtain thinner sections of the original slices. However, this will result in increase the incidence of artifacts in staining as well as alter the integrity of the tissue.
II. Materials/equipment
Preparation of glass bottom dishes for organotypic slices
- Small syringes (1 mL)
- 23-gauge needles
- Nucleopore Track-Etch Membrane - diameter 25mm, pore size 8.0μm - Whatman, cat# 110614
- Glass Bottom Culture Dishes - 35mm petri dish, 14mm Microwell, No. 1.5 coverglass - MatTech, cat# P35G-1.5-14-C
- Rubber Cement - Elmer's, cat# E904
- Basal Medium Eagle - Gibco, cat# 21010
- 1M Hepes (pH7.4)
- 1M D-glucose
- 100mM CaCl2
- 100mM MgSO4
- 1M NaHCO3
- dH2O
- 200mM L-glutamine
- Penicillin-streptomycin
Brain extraction and embedding
- Anesthetic (isofluorane, etc.)
- Microwave oven
- Low Melting Agarose - Fisher, cat# BP1360-100
- Krazy glue - cat#KG585
- Peel-A-Way Disposable Embedding Molds (R-40) - 22mmx40mm rectangular, 20mm deep - Polysciences, cat# 18646C
Brain sectioning and RMS transplantation
- Vibratome - Leica VT1000S and all accessory components for slice preparation
- 10X Hank's Balanced Salt Solution - Gibco, cat# 14185
- Microdissecting forceps #5 - Roboz, cat# RS-4976
- Microspatula - Fisher, cat# 21-401-15
- Stereomicroscope
Time-lapse imaging of organotypic slices
- Humidified Incubator, 5% CO2
- Inverted Microscope equipped with incubator chamber and long distance working objectives (NA of 0.6 or higher)
III. Recipes
Buffer solution for tissue dissection and slice preparation (tissue preparation buffer)
| Stock solution | Volume | Final Concetration |
| 10X HBSS | 50 mL | 1X |
| 1M Hepes (pH 7.4) | 1.25 mL | 2.5mM |
| 1M D-Glucose | 15 mL | 30mM |
| 1M CaCl2 | 0.5 mL | 1mM |
| 1M MgSO4 | 0.5 mL | 1mM |
| 1M NaHCO3 | 2 mL | 4mM |
| dH2O | 430.75 mL |
Filter sterilize with a 0.2 μm filter and store at 4°C.
Culture medium for organotypic slices, tissue transplantation and imaging (slice medium)
| Stock solution | Volume | Final Concentration |
| Basal Medium Eagle | 35 mL | |
| Tissue preparation buffer | 12.9 mL | |
| 1M D-Glucose | 1.35 mL | 20mM |
| 200mM L-glutamine | 0.25 mL | 1mM |
| Penicillin-streptomycin | 0.5 mL | 100units/ mL penicillin and 0.1mg/ mL streptomycin |
Filter sterilize with a 0.2 μm filter and store at 4°C.
Preparation of Low-melting point agarose gel
Low-melting-point agarose is diluted in tissue preparation buffer at 0.3g/ mL in a 50 mL conical tube (see recipes). The tube is microwaved in increments of 5-10 seconds at high-power. Number of increments is dependent on total volume; for 10 mL, three increments (10-8-5 seconds, each) should suffice. The tube cap is carefully unscrewed between heating increments to release air pressure and avoid explosion of the tube. Caution must be taken as the tube content will be very hot. Once the agarose is completely dissolved, the tube is maintained in a 37°C water bath for at least 5 minutes to allow the temperature to stabilize prior to use. Prolonged exposure to room temperature will harden the gel. Although this must be avoided as much as possible, hardened gel can be reheated and re-melted for immediate use within 24 hours of initial preparation.
Immunohistochemistry on organotypic slices
Following imaging on the confocal microscope, slices may be fixed overnight at 4°C with 4% formaldehyde in PBS. Sections are then blocked overnight at 4°C, in 10% goat serum with 1% Triton X (Sigma, Cat. # S26-36-23) in PBS followed by overnight incubation with primary antibodies at 4°C. Fluorescently-tagged goat secondary antibodies are used for visualization (all diluted 1:1000, 1 hour incubation at room temperature). Labeled slices are thoroughly washed 5-6 times with ice cold PBS prior to mounting on glass slides and coverslipping.
IV. Representative Results
Our organotypic slice culture protocol has been thoroughly tested and optimized over that last few years for consistency in migration pattern and orientation. Analysis of cells emigrating from explants obtained from mice in which the expression of the red fluorescent protein, Td-tomato, is induced under the Nestin promoter (Nestin-Td tomato), reveals highly oriented and rapid migration of tdTomato+ neuroblasts into the host RMS (Figure 4A). High-magnification time-lapse analysis illustrates excellent resolution of the entire length of a migrating neuroblast during a 20-minute imaging session (Figure 4B).
Slices with donor Td-tomato+ cells were fixed and immunostained for different cellular components within the RMS. GFAP+ astrocytes and CD31+ blood vessels were revealed using fluorescent immunohistochemistry (Figure 5A). High-magnification analysis of slices stained for the cytoskeletal proteins actin and tubulin reveal non-uniform expression of these components by a cell in the midst of migration (Figure 5B).
Antibodies used in these examples: rabbit anti-RFP (Abcam, 1:250), rabbit anti-GFAP (Dako, 1:1000), rat anti-CD31(BD Pharmigen, 1:100), mouse anti-actin (Santa Cruz, 1:500), rabbit anti-tubulin (Sigma, 1:1000), goat anti-mouse Cy3 (Chemicon, 1:1000), goat anti-rabbit AlexaFluor 647 (Invitrogen, 1:1000), goat anti-rat AlexaFluor 488 (Invitrogen, 1:1000), goat anti-rabbit AlexaFluor 488 (Invitrogen, 1:1000).
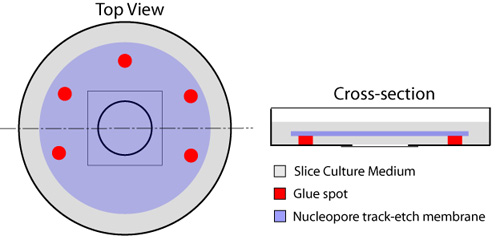
Figure 1. Preparation of glass-bottom dishes for organotypic slices. Multiple spots of glue are placed around the circular glass-bottom component of the dish (red), leaving one side open for exchange of medium from underneath the filter. A 150μL drop of slice medium is placed in the center of the glass coverslip. A nucleopore membrane (blue) is then applied, shiny side down, while ensuring that no air bubbles are trapped between the glass coverslip and the membrane. One milliliter of slice medium (grey) is spread on top of membrane, and dishes are incubated at 37° C prior to use.
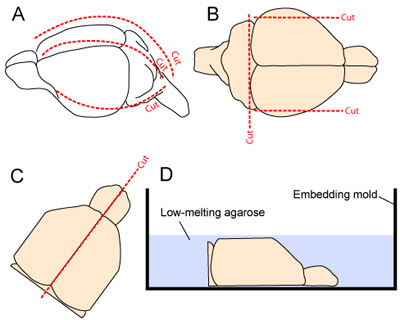
Figure 2. Brain extraction and preparation for sectioning. (A) The skull is exposed by incising the scalp from the neck to the snout (dotted line along the midline). The skull is then cut longitudinally and anteriorly starting at the cisterna magna, by making 1 medial and 2 lateral cuts (one on each side; 2A). (B) The lateral-most aspects of the cortex and the caudal aspect of the CNS are resected to improve stability of the tissue during vibratome sectioning. (C-D) The two hemispheres are then separated and placed medial face down in an embedding mold prior to application of 3% agarose gel dissolved in tissue preparation buffer.
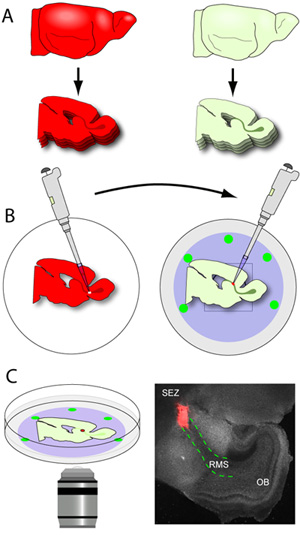
Figure 3. Brain sectioning and cross-transplantation. (A) The host tissue is sectioned at 150μm thickness, and the RMS-containing sections are carefully positioned flat on the nucleopore membrane of cold glass-bottom dishes. (B) Donor brains (brains expressing fluorescent reporters in the RMS) are sectioned at 250μm thickness, and the slices are collected in ice-cold tissue preparation buffer. The donor RMS is microdissected and cut into small explants. Using a pipettor equipped with a 20 μL tip, individual RMS explants are transferred to an incised site in the host RMS. (C) After 1-2 hours of incubation, dishes are transferred to an incubated stage on a confocal microscope and migration is captured using time-lapse imaging. The photomicrograph is a representative low-magnification image of a typical slice (grey) set up 1 hour after transplantation (red explant from tdTomato+ RMS of a donor mouse; red dotted lines outline the RMS in the host slice).
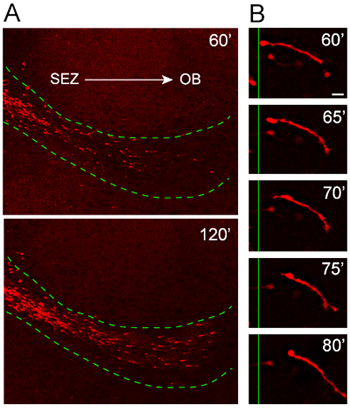
Figure 4. Migration of neuroblasts from explants into the host RMS. (A) Nestin-tdTomato+ neuroblasts (red) migrate from the explants into the host RMS (dotted green line) 1 hour after transplantation. tdTomato+ cells invading the RMS of the host organotypic slices move in a highly oriented and rapid manner away from the SEZ and toward the OB. (B) The migratory cycle can be observed in high-power time-lapse images of a neuroblast over approximately a 20 minute period. Scale bar = 10 μm.
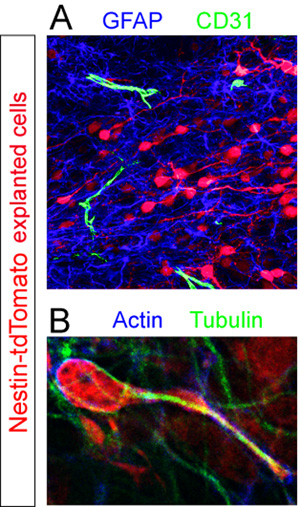
Figure 5. Immunohistochemical assessment of organotypic slices. Explanted neuroblasts (red) were fixed in the midst of migration 12 hours post transplantation. (A) Fluorescent immunohistochemical staining of the slice reveals a dense pool of GFAP+ astrocytes (blue) and scattered CD31+ blood vessels (green) within the RMS of the host slice. (B) The cytoskeleton of an isolated tdTomato+ migrating cell (red) in a host RMS is revealed by co-immunostaining using antibodies against actin (blue) and tubulin (green).
Discussion
Neuronal migration in the RMS is an essential component of postnatal neurogenesis in the olfactory bulbs 1. Migration through the RMS occurs in a plane tangential to the surface of the brain. Tangentially migrating neuroblasts are distinct from radially migrating cells based on the location of their progenitor source, as well as the divergent fate of their final neuronal products 1, 2, 3. The relatively pure population of tangentially migrating cells in the postnatal RMS makes this anatomically de...
Disclosures
Experiments on animals were performed in accordance with the guidelines and regulations set forth by North Carolina State University and its College of Veterinary Medicine. No conflicts of interest declared.
Acknowledgements
We thank Dan McWhorter for narrating the protocol in the video. This work is supported by NIH Grant 5R01NS062182, a grant from American Federation for Aging Research, and institutional funds awarded to HTG.
References
- Ghashghaei, H. T., Lai, C., Anton, E. S. Neuronal migration in the adult brain: are we there yet. Nat. Rev. Neurosci. 8, 141-151 (2007).
- Valiente, M., Marin, O. Neuronal migration mechanisms in development and disease. Curr. Opin. Neurobiol. 20, 68-78 (2010).
- Rakic, P. Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 10, 724-735 (2009).
- Jaglin, X. H., Chelly, J. Tubulin-related cortical dysgeneses: microtubule dysfunction underlying neuronal migration defects. Trends Genet. 25, 555-566 (2009).
- Carro, M. S. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 463, 318-325 (2010).
- Wu, W. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 400, 331-336 (1999).
- Hu, H., Tomasiewicz, H., Magnuson, T., Rutishauser, U., U, . The role of polysialic acid in migration of olfactory bulb interneuron precursors in the subventricular zone. Neuron. 16, 735-743 (1996).
- Shapiro, E. M., Gonzalez-Perez, O., Garcia-Verdugo, M. a. n. u. e. l., Alvarez-Buylla, J., &, A., Koretsky, A. P. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. Neuroimage. , (2006).
- Vreys, R. MRI visualization of endogenous neural progenitor cell migration along the RMS in the adult mouse brain: validation of various MPIO labeling strategies. Neuroimage. 49, 2094-2103 (2010).
- Davenne, M., Custody, C., Charneau, P., Lledo, P. M. In vivo imaging of migrating neurons in the mammalian forebrain. Chem. Senses. 30, 115-116 (2005).
- Mirzadeh, Z., Doetsch, F., Sawamoto, K., Wichterle, H., Alvarez-Buylla, A. The subventricular zone en-face: wholemount staining and ependymal. J. Vis. Exp. , (2010).
- Shen, Q. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 3, 289-300 (2008).
- Tavazoie, M. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 3, 279-288 (2008).
- Mirzadeh, Z., Merkle, F. T., Soriano-Navarro, M., Garcia-Verdugo, J. M., Alvarez-Buylla, A. Neural Stem Cells Confer Unique Pinwheel Architecture to the Ventricular Surface in Neurogenic Regions of the Adult Brain. Cell Stem Cell. 3, 265-278 (2008).
- Polleux, F. &. a. m. p. ;. a. m. p., Ghosh, A. The slice overlay assay: a versatile tool to study the influence of extracellular signals on neuronal. Sci. STKE. , L9-L9 (2002).
- Murase, S. &. a. m. p. ;. a. m. p., Horwitz, A. F. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J. Neurosci. 22, 3568-3579 (2002).
- Suzuki, S. O. &. a. m. p. ;. a. m. p., Goldman, J. E. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J. Neurosci. 23, 4240-4250 (2003).
- Ghashghaei, H. T. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc. Natl. Acad. Sci. U. S. A. 103, 1930-1935 (2006).
- Khodosevich, K., Seeburg, P. H., Monyer, H. Major signaling pathways in migrating neuroblasts. Front Mol. Neurosci. 2, 7-7 (2009).
- Jacquet, B. V. Analysis of neuronal proliferation, migration and differentiation in the postnatal brain using equine infectious anemia virus-based lentiviral vectors. Gene Ther. 16, 1021-1033 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved