Method Article
Expansion, Purification, and Functional Assessment of Human Peripheral Blood NK Cells
In This Article
Summary
Here we describe a method to efficiently expand and purify large numbers of human NK cells and assess their function.
Abstract
Natural killer (NK) cells play an important role in immune surveillance against a variety of infectious microorganisms and tumors. Limited availability of NK cells and ability to expand in vitro has restricted development of NK cell immunotherapy. Here we describe a method to efficiently expand vast quantities of functional NK cells ex vivo using K562 cells expressing membrane-bound IL21, as an artificial antigen-presenting cell (aAPC).
NK cell adoptive therapies to date have utilized a cell product obtained by steady-state leukapheresis of the donor followed by depletion of T cells or positive selection of NK cells. The product is usually activated in IL-2 overnight and then administered the following day 1. Because of the low frequency of NK cells in peripheral blood, relatively small numbers of NK cells have been delivered in clinical trials.
The inability to propagate NK cells in vitro has been the limiting factor for generating sufficient cell numbers for optimal clinical outcome. Some expansion of NK cells (5-10 fold over 1-2 weeks) has be achieved through high-dose IL-2 alone 2. Activation of autologous T cells can mediate NK cell expansion, presumably also through release of local cytokine 3. Support with mesenchymal stroma or artificial antigen presenting cells (aAPCs) can support the expansion of NK cells from both peripheral blood and cord blood 4. Combined NKp46 and CD2 activation by antibody-coated beads is currently marketed for NK cell expansion (Miltenyi Biotec, Auburn CA), resulting in approximately 100-fold expansion in 21 days.
Clinical trials using aAPC-expanded or -activated NK cells are underway, one using leukemic cell line CTV-1 to prime and activate NK cells5 without significant expansion. A second trial utilizes EBV-LCL for NK cell expansion, achieving a mean 490-fold expansion in 21 days6. The third utilizes a K562-based aAPC transduced with 4-1BBL (CD137L) and membrane-bound IL-15 (mIL-15)7, which achieved a mean NK expansion 277-fold in 21 days. Although, the NK cells expanded using K562-41BBL-mIL15 aAPC are highly cytotoxic in vitro and in vivo compared to unexpanded NK cells, and participate in ADCC, their proliferation is limited by senescence attributed to telomere shortening8. More recently a 350-fold expansion of NK cells was reported using K562 expressing MICA, 4-1BBL and IL159.
Our method of NK cell expansion described herein produces rapid proliferation of NK cells without senescence achieving a median 21,000-fold expansion in 21 days.
Protocol
1. Isolation of PBMCs from Buffy Coat
Peripheral blood mononuclear cells (PBMC) are obtained by buoyant density centrifugation on Ficoll-Paque from healthy donor buffy coat samples derived by leukapheresis.
- The Ficoll-Paque centrifugation is done as per manufacturer's protocol with minor modifications.
- Add PBS to a normal-donor blood-bank buffy coat to a final volume of 140 mL (typical buffy coat volume is 40-70 mL).
- Layer 35 mL of buffy coat sample on 15 mL of Ficoll-Paque (4 tubes).
- Centrifuge at 400g for 20 minutes without brake.
- Recover the PBMCs from the Ficoll-Paque: plasma interface, do not discard the RBCs at the bottom of the Ficoll-Paque.
- Wash PBMCs three times with PBS, centrifuging each time at 400g for 10 minutes.
- PBMCs can be used directly for NK cell expansion at this stage or NK cells can be isolated by RosetteSep (Section 4)
- Remaining PBMCs can be frozen in FBS containing 10% DMSO in liquid nitrogen.
- Aspirate the Ficoll and collect the RBCs from step 5 in to two 50 mL centrifuge tube, wash three times with PBS (added to 50 mL mark), each time aspirate the supernatant skimming the top of the RBC layer to remove granulocytes.
- The RBCs can be used immediately for RosetteSep purification of NK cells (refer to Section 4) or stored in equal volume of Alsever's solution at 4°C for later use (The RBCs can be store for a maximum of 4 weeks).
2. NK Cell Expansion
The NK cell expansion can be initiated using PBMCs or purified NK cells. The amount of PBMCs used for expansion can be varied based on the amount of NK cells desired at the end of a three week expansion, refer to representative results section for details. (See Note 1)
STIMULATION 1
Day 0
- For each 5x106 PBMCs to be expanded, count and irradiate 10x106 K562 Cl9 mIL21 using a gamma irradiator at 100 Gy.
- Post irradiation, wash the cells with PBS and resuspend in NK cell expansion media (NKEM).
- Seed 5x106 PBMCs with 10x106 irradiated K562 Cl9 mIL21 (1:2 ratio) in 40 mL of NKEM in a T75 flask and place it upright in an incubator at 37°C and 5% CO2.
Days 3 and 5
- Recover cells by centrifugation at 400g for 5 min and replace half of the media with fresh NKEM (adding fresh IL2 for the entire media volume) and continue culture.
STIMULATION 2
Day 7
- Count the number of cells in culture at the end of one week.
- Set aside 5x105 cells for phenotyping by flow cytometry (See Note 2)
- For each 5x106 cells to be restimulated, count and irradiate 5x106 K562 Cl9 mIL21 using a gamma irradiator at 100 Gy.
- Add an equal number of irradiated K562 Cl9 mIL21 (1:1 ratio) and resuspend in NKEM at 2.5x105 total cells/mL (See Note 3).
- Seed cells in T75 flasks (maximum 50 mL per flask).
Days 10 and 12
- Count the number of cells.
- Change entire media with fresh NKEM based on the cell numbers (See Note 3).
Day 14
- At the end of two weeks of expansion count the number of cells in culture.
- Set aside 5x105 cells for phenotyping by flow cytometry (See Note 2)
- If expansion was started from PBMCs the NK cells can be purified at this stage of expansion using the RosetteSep purification protocol (refer to Section IV). If expansion was started from purified NK cells proceed to stimulation 3.
- After purification set aside 5x105 cells for phenotyping by flow cytometry to verify purity of the NK cells (as in Step 8).
- Proceed with Stimulation 3 using all of the purified NK cells (See Note 4).
STIMULATION 3
- Resuspend NK cells with irradiated K562 Cl9 mIL21 (1:1 ratio) in NKEM based on cell numbers (See Note 3).
Days 17 and 19
- Count the number of cells.
- Change media with fresh NKEM based on the cell numbers (See Note 3).
Day 21
- At the end of three weeks of expansion count the number of cells in culture.
- Recover 1x106 cells for flow cytometry analysis for full NK cell phenotyping antibody panel (See Table 1).
- Freeze cells in FBS containing 10% DMSO at a maximum density of 5x107 cells per vial for future use.
3. NK Cell Cytotoxicity Assay
- Thaw a vial of NK cells and seed in NKEM, one day prior to performing cytotoxicity assay to allow recovery.
- For each NK cell cytotoxicity assay using a single target cell line, 6x105 NK cells and 3x105 target cells are required (See Note 5).
- Prepare CAM-Media by diluting Calcein-AM (stock 1 mg/mL in DMSO) in NKEM (See Note 6).
- Resuspend 106 target cells in 1 mL of CAM-media (See Note 7).
- Incubate for 1 h at 37 °C, with occasional shaking.
- Resuspend NK cells at 1x106 cells/ mL and add 200uL of NK cell suspension to each of the 3 wells of a U-bottom 96-well plate corresponding to10:1 E:T ratio shown in Figure 1. (See Note 8)
- Add 100uL of complete media to all remaining wells except for "Maximum".
- Add 100uL of 2% Triton X-100 to "Maximum".
- Perform serial dilutions of the NK cells for the 5 subsequent E:T ratios by transferring 100uL of cells each time, mix well. Discard 100uL from the last wells (E:T ratio of 0.3125:1).
- After 1 hour of calcein loading, wash target cells in NKEM twice, centrifuging for 5 min at 1200 rpm. (See Note 9)
- Re-count the target cells and resuspend at 1x105 cells/ mL.
- Add 100 μL of target cells to each well (1x104/well). Centrifuge for 1 min at 100g to initiate cell contact.
- Incubate at 37 °C and 5% CO2 for 4 hours.
- Mix the culture gently by pipetting with a 100 μL pipetter in order to uniformly suspend the released calcein, spin down plate at 100g for 5 minutes to pellet the cells and transfer 100 μL of the supernatant to a new plate taking care to avoid bubbles. Pop any bubbles that may form using fine needle.
- Read the plate using a fluorescent plate reader (excitation filter 485 nm, emission filter 530 nm). Bottom read is recommended.
- Calculate Percent Specific Lysis according to the formula [(test release-spontaneous release)/(maximum release - spontaneous release)] x 100.
4. NK Cell Purification by RosetteSep
- Take 100-fold excess of RBCs to that of PBMCs or expanded cells, into a 50 mL tube (100:1 RBC:PBMC).
- If using fresh RBCs proceed directly to next step or if the RBCs were stored in Alsever s solution, count the number of RBCs and wash appropriate (100 fold excess) amount of RBCs with PBS supplemented with 2% FBS three times, centrifuging at 1200 rpm for 10 minutes each time.
- Combine RBCs with PBMCs from step 1.7 or expanded cells from step 2.10 in PBS + 2% FBS to a final volume of 1 mL per 5x107 of PBMCs or expanded cells.
- Add 1μL of RosetteSep Human NK Cell Enrichment Cocktail per 1x106 of PBMCs or expanded cells.
- Mix well and incubate at room temperature for 20 minutes with gentle mixing every 5 minutes.
- Add equal volume of PBS + 2% FBS mix gently and layer on top of Ficoll-Paque.
- Repeat the steps of Ficoll-Paque centrifugation described for PBMC isolation (Section I).
- Count the NK cells recovered after purification and set aside 5x105 cells for phenotpying by flow cytometry for NK cell purity (Step 8).
5. Notes
NOTE 1. NK cells can be expanded directly from PBMCs, or from RosetteSep purified NK cells. We have noted similar expansion efficiency, but some donors may have very low NK cell numbers resulting in difficulty purifying by RosetteSep prior to expansion.
NOTE 2. We routinely use CD56-FITC, CD16-PE, and CD3-PE-Cy5 for phenotyping during the expansion, enumerating NK cells as those which are CD3-negative and CD16- or CD56-positive.
NOTE 3. At each media change or stimulation, resuspend cells at 2.5 x 105/mL to keep PBMC/NK cell numbers at or under 2 million per mL at peak stages of expansion. This will prevent depletion of nutrients and help achieve maximal expansion and survival.
NOTE 4. The NK expansion rate is donor dependent and at the end of stimulations 1 or 2 part of the cells can be frozen and part expanded further depending on the experimental need. We have had good success in using the frozen cells for expansions at a later time.
NOTE 5. In order to allow room for error we recommend using a minimum of 7x105 NK cells resuspended in 700 uls of NKEM and 4x105 Calcein-AM stained target cells resuspended in 4 mL of NKEM for setting up the cytotoxicity assay. If using multichannel pipette for seeding target cells higher volumes of cells (up to 6x105 in 6 mL) may be required based on the size of media basin being used. Also the recommended NK cell numbers are specifically for the E:T ratios show in the protocol, for using higher E:T ratios increase the NK cell numbers per mL accordingly (eg. For a 40:1 E:T ratio use 4x106 cells/ mL)
NOTE 6. We recommend performing a preliminary Calcein-AM loading titration for the target cell line of choice, using the following dilutions of 1:500, 1:400. 1:300, 1:200 and 1:100 to achieve optimal difference between maximum and spontaneous release.
NOTE 7. When using an adherent cell line as target, first prepare single cell suspension using non-enzymatic cell dissociation buffer. If performing ADCC, prepare a duplicate tube of target cells in CAM-media.
NOTE 8. If performing ADCC, add same NK cells to 3 wells corresponding to 10:1 E:T for ADCC. Repeat for additional donors. Repeat for additional target cells.
NOTE 9. If performing an ADCC experiment, after 45 minutes of calcein loading, add 10ug of antibody specific for inducing ADCC against the target cells. After 15 more minutes, wash target cells in complete medium twice, centrifuging for 5 min at 1200 rpm. Resuspend cells at 1x105 cells/ mL and proceed with the next step in the protocol.
6. REPRESENTATIVE RESULTS
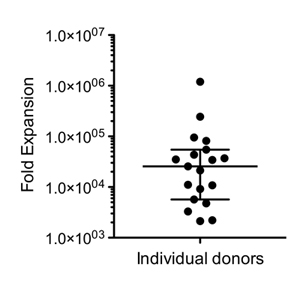
Figure 2. When the expansion is performed as per the scheme described above, using 5x106 PBMCs as starting material, typical NK cell yields range from 1x109 to 1010 cells (donor dependent variability). The figure shows NK cell fold expansion (n=19) compared to NK cells present in the original product (median +/- quartile).
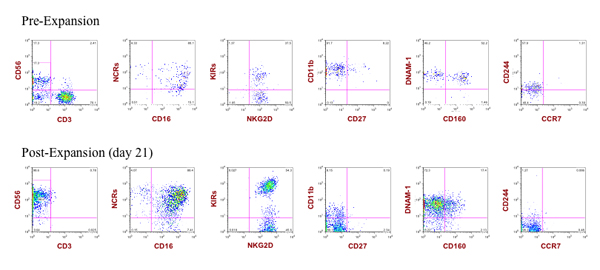
Figure 3. The expanded NK cells express various NK cell receptors that are comparable to the unexpanded primary NK cells with a few exceptions (CD11b, CD160 and CD244).

Figure 4. PBMCs recovery from Buffy coat is donor dependent and can range from 300x106 to 800x106. NK cells may comprise 2% - 18% of the PBMCs. For RosetteSep purification of expanded cells, recovery of pure NK cells on day 14 ranges from 40-70%. By following the recommended protocol of expansion and purification, NK cell purity of 99% can be expected.

Figure 5. Expanded NK cells have demonstrated cytotoxicity against a range of tumor cell lines including neuroblastoma, AML, osteosarcoma and melanoma (representative AML killing shown as percent specific lysis).
Disclosures
No conflicts of interest declared.
Acknowledgements
The authors would like to thank Laurence Cooper, Harjeet Singh, and Lenka Hurton for their work in creating the initial K562 aAPC and mIL21 fusion vectors.
Funding for this work was provided by the UT MD Anderson Physician Scientist Program, the St. Baldrick's Foundation, and the Legends of Friendswood.
Materials
| Name | Company | Catalog Number | Comments |
NK Cell Expansion and Activation Media (NKEM) | |||
| 90% RPMI 1640 | Cellgro | ||
| 10% Fetal Bovine Serum | Gibco | ||
| 1x Penicillin / Streptomycin | Cellgro | ||
| 1x L-Glutamine | Gibco | Filter Sterilize media before use. | |
| 50 U/ mL IL2 | Proleukin, Novartis Vaccines and Diagnostics, Inc) | Diluted from a 200 IU/μl stock. Add IL2 to desired amount of media just before use each time. | |
| [header] | |||
PBMC and NK Cell Isolation | |||
| Ficoll-Paque | GE Healthcare | ||
| Alsever's solution | Sigma) | ||
| RosetteSep Human NK Cell Enrichment Cocktail | Stemcell Technologies) | ||
| [header] | |||
NK Cell Cytotoxicity Assay | |||
| Calcein-AM | Invitrogen | ||
| [header] | |||
Antibodies | |||
The list of antibodies used for NK cell phenotyping are listed in table below: | |||
Tube 1: (total volume 100) | |||
| Antibody: Isotype FITC | BD Pharmingen | 555748 | Volume: 5 |
| Antibody: Isotype FITC | BD Pharmingen | 555749 | Volume: 5 |
| Antibody: Isotype FITC | BD Pharmingen | 557224 | Volume: 5 |
| Antibody: Isotype FITC | BD Pharmingen | 340442 | Volume: 5 |
| Antibody: FACS Buffer | BD Pharmingen | Volume: 80 | |
Tube 2: (total volume 100) | |||
| Antibody: CD56 FITC | BD Pharmingen | 340410 | Volume: 5 |
| Antibody: NKp30 PE | BD Pharmingen | 558407 | Volume: 5 |
| Antibody: NKp44 PE | BD Pharmingen | 558563 | Volume: 5 |
| Antibody: NKp46 PE | BD Pharmingen | 557991 | Volume: 5 |
| Antibody: CD3 PE-Cy5 | BD Pharmingen | 555341 | Volume: 5 |
| Antibody: CD16 Alexa 647 | BD Pharmingen | 557710 | Volume: 5 |
| Antibody: FACS Buffer | BD Pharmingen | Volume: 70 | |
Tube 3: (total volume 100) | |||
| Antibody: CD56 FITC | BD Pharmingen | 340410 | Volume: 5 |
| Antibody: KIR2DL1 PE | R&D Systems | FAB1844P | Volume: 5 |
| Antibody: KIR2DL2/3 PE | Miltenyi Biotec | 130-092-618 | Volume: 5 |
| Antibody: KIR3DL1 PE | R&D Systems | FAB12251P | Volume: 5 |
| Antibody: CD3 PE-Cy5 | BD Pharmingen | 555341 | Volume: 5 |
| Antibody: NKG2D APC | BD Pharmingen | 558071 | Volume: 5 |
| Antibody: FACS Buffer | BD Pharmingen | Volume: 70 | |
Tube 4: (total volume 100) | |||
| Antibody: CD56 FITC | BD Pharmingen | 340410 | Volume: 5 |
| Antibody: CD11b PE | BD Pharmingen | 555388 | Volume: 5 |
| Antibody: CD3 PE-Cy5 | BD Pharmingen | 555341 | Volume: 5 |
| Antibody: CD27 APC | BD Pharmingen | 558664 | Volume: 5 |
| Antibody: FACS Buffer | BD Pharmingen | Volume: 80 | |
Tube 5: (total volume 100) | |||
| Antibody: CD56 FITC | BD Pharmingen | 340410 | Volume: 5 |
| Antibody: CD266 (DNAM-1) PE | BD Pharmingen | 559789 | Volume: 5 |
| Antibody: CD3 PE-Cy5 | BD Pharmingen | 555341 | Volume: 5 |
| Antibody: CD160 Alexa647 | eBiosciences | 51-1609-42 | Volume: 5 |
| Antibody: FACS Buffer | BD Pharmingen | Volume: 80 | |
Tube 6: (total volume 100) | |||
| Antibody: CD56 FITC | BD Pharmingen | 340410 | Volume: 5 |
| Antibody: CD244 (2B4) PE | BD Pharmingen | 550816 | Volume: 5 |
| Antibody: CD3 PE-Cy5 | BD Pharmingen | 555341 | Volume: 5 |
| Antibody: CD197 (CCR7) APC | eBiosciences | 17-1979-42 | Volume: 5 |
| Antibody: FACS Buffer | BD Pharmingen | Volume: 80 | |
References
- McKenna, D. H. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 47, 520-520 (2007).
- Koehl, U. ex vivo expansion of highly purified NK cells for immunotherapy after haploidentical stem cell transplantation in children. Klinische Padiatrie. 217, 345-345 (2005).
- Klingemann, H. G., Martinson, J. ex vivo expansion of natural killer cells for clinical applications. Cytotherapy. 6, 15-15 (2004).
- Ayello, J. Characterization of cord blood natural killer and lymphokine activated killer lymphocytes following ex vivo cellular engineering. Biol Blood Marrow Transplant. 12, 608-608 (2006).
- Carlens, S. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum Immunol. 62, 1092-1092 (2001).
- Boissel, L. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant. 14, 1031-1031 (2008).
- North, J. Tumor-primed human natural killer cells lyse NK-resistant tumor targets: evidence of a two-stage process in resting NK cell activation. J Immunol. 178, 85-85 (2007).
- Berg, M. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 11, 341-341 (2009).
- Fujisaki, H. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 69, 4010-4010 (2009).
- Fujisaki, H. Replicative potential of human natural killer cells. Br J Haematol. 145, 606-606 (2009).
- Gong, W. Ex vivo expansion of natural killer cells with high cytotoxicity by K562 cells modified to co-express major histocompatibility complex class I chain-related protein A, 4-1BB ligand, and interleukin-15. Tissue Antigens. 76, 467-467 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved