A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Engineering Biological-Based Vascular Grafts Using a Pulsatile Bioreactor
In This Article
Summary
Our group has developed a bioreactor culture system that mimics the physiological pulsatile stresses of the cardiovascular system to regenerate implantable small-diameter vascular grafts.
Abstract
Much effort has been devoted to develop and advance the methodology to regenerate functional small-diameter arterial bypasses. In the physiological environment, both mechanical and chemical stimulation are required to maintain the proper development and functionality of arterial vessels1,2.
Bioreactor culture systems developed by our group are designed to support vessel regeneration within a precisely controlled chemo-mechanical environment mimicking that of native vessels. Our bioreactor assembly and maintenance procedures are fairly simple and highly repeatable3,4. Smooth muscle cells (SMCs) are seeded onto a tubular polyglycolic acid (PGA) mesh that is threaded over compliant silicone tubing and cultured in the bioreactor with or without pulsatile stimulation for up to 12 weeks. There are four main attributes that distinguish our bioreactor from some predecessors. 1) Unlike other culture systems that simulate only the biochemical surrounding of native blood vessels, our bioreactor also creates a physiological pulsatile environment by applying cyclic radial strain to the vessels in culture. 2) Multiple engineered vessels can be cultured simultaneously under different mechanical conditions within a controlled chemical environment. 3) The bioreactor allows a mono layer of endothelial cells (EC) to be easily coated onto the luminal side of engineered vessels for animal implantation models. 4) Our bioreactor can also culture engineered vessels with different diameter size ranged from 1 mm to 3 mm, saving the effort to tailor each individual bioreactor to fit a specific diameter size.
The engineered vessels cultured in our bioreactor resemble native blood vessels histologically to some degree. Cells in the vessel walls express mature SMC contractile markers such as smooth muscle myosin heavy chain (SMMHC)3. A substantial amount of collagen is deposited within the extracellular matrix, which is responsible for ultimate mechanical strength of the engineered vessels5. Biochemical analysis also indicates that collagen content of engineered vessels is comparable to that of native arteries6. Importantly, the pulsatile bioreactor has consistently regenerated vessels that exhibit mechanical properties that permit successful implantation experiments in animal models3,7. Additionally, this bioreactor can be further modified to allow real-time assessment and tracking of collagen remodeling over time, non-invasively, using a non-linear optical microscopy (NLOM)8. To conclude, this bioreactor should serve as an excellent platform to study the fundamental mechanisms that regulate the regeneration of functional small-diameter vascular grafts.
Protocol
Autoclave
Assemble and autoclave the tubing for the flow system and bioreactor components (bioreactor itself and the silicone stopper lid) as instructed in Figure 1 and Figure 2. Feeding tube has a male connector on one end and an open end on the other side. Three short tubing segments are inserted through a silicone cap for gas exchange.
1. Sewing PGA Mesh
- Cut PGA mesh to 1.1cm x ~8cm sheet (dependent on bioreactor size).
- Clean silicone tubing (3mm inner diameter) with distilled water (dH20) and air dry before usage.
- Use Dexon 6.0 suture to sew PGA mesh around the clean silicone tubing starting with three surgical knots followed by single stitches.
2. PGA Scaffolds Surface Treatment
- Dip PGA scaffolds in 1M NaOH for 1-2min and record the treatment time and use the same time for all PGA scaffolds.
- Rinse PGA scaffolds in dH2O baths for 2 minutes 3 times.
- Pat PGA scaffolds dry with Kimwipes between each dip in dH2O baths.
- Dry PGA scaffolds under tissue culture hood to air dry for 15 minutes with blower on.
3. Sewing Dacron arms
- Use Prolene 4.0 suture to sew small pieces of Dacron cuffs (1cm) onto each end of the PGA mesh with an overlap of 2-3mm. Note: be careful not to puncture silicone tubing (Figure 3).
- Use the same suture to sew three stitches around the free end of Dacron cuffs. Make sure to leave enough sutures at the free ends of Prolene suture for step 5.1 (Figure 3).
4. Assembly of Bioreactor (Day before beginning of bioreactor culture)
- Soak sewn mesh and silicone tubing, surgical instruments, and a thin wire in a 70% ethanol bath for 20-30 minutes.
- Open the autoclave pouches containing the bioreactor and submerge it in 70% ethanol bath for at least 30 minutes prior to assembling. Make sure to flush the bioreactor thoroughly with ethanol. Exposure of all bioreactor components to 70% ethanol is an addition sterilization step. This step also removes endotoxin, which is not removed by autoclaving and is deleterious to the vascular cells.
- Pull silicone tubing through side-arms using thin wire to pull it through.
- Take bioreactor out of ethanol bath (keep mesh submerged in ethanol). Fix the PGA scaffold inside the bioreactor by fastening Dacron cuffs over the flared glass lips by tightening and tying down the Prolene sutures (Figure 3).
- Connect one side of bioreactor side arms to connectors via silicone tubing.
- Pull other side of silicone tubing with enough tension and insert the remaining two connectors into silicone tubing. Hold on to silicone tubing tightly when inserting connectors!
- Reinsert bioreactor into ethanol bath and flush with ethanol by gently pulling connectors out of the side arms.
- Flip bioreactor over and allow a soak for 10 minutes.
- Flip bioreactor right-side up and soak for an additional 10 minutes.
- Drain all ethanol.
- Set up three large petri dishes (10 cm) in series and place bioreactor in the center dish to hold the excess ethanol dripped from the ends of the tubes.
- Flush bioreactor and PGA mesh with tissue culture water using a 5ml or 10ml pipette. Also flush tissue culture water into silicone tubing.
- Thoroughly drain all excess water into petri dishes on either side of the bioreactor.
- Dry bioreactor overnight in hood with blower on and UV OFF.
- Additional Notes: make sure sterile stir bar is in bioreactor. Make sure not to "hover" over the bioreactor from this point forward, to avoid contamination. Cut up plenty of parafilm strips and soak them in a small 70% ethanol bath (large petri dish works well).
5. Day 1: Bioreactor Setup
- Place a sterile petri dish over opening of each bioreactor to protect the PGA scaffold inside from contaminants.
- Assemble flow system to bioreactor as indicated in Figure 4 and parafilm all the connection joints.
- Wipe connectors first with alcohol wipes.
- Attach injection port to third, unused arm of bioreactor.
- Remove walrus tubing and tie off blue end of saline dilution set as close to Y-junction as possible. Pull tube clamp in place to ensure no liquid transfer to this part of the tube
- Remove IV bag, & attach walrus tubing (red) to far end of IV bag. Make sure to wipe insertion port with alcohol wipe first.
- Attach walrus to one side of flow system (via white end tube).
- Insert 3-way stopcock into flow system.
- Remove pressure transducer & connect to three-way stopcock.
- Attach other end of pressure transducer to middle opening in bag.
- Connect the bioreactor to the flow system via the Y-junction. Use a 60ml syringe to add 350ml of 1% fungizone (mix 5ml fungizone with 495ml of PBS) to IV bag.
- Squeeze IV bag to flush the flowing system by adjusting the stop cocks to allow flow of PBS. Note: check inside bioreactor to ensure there is no leaking.
- Re-suspend 8 x106 SMCs (about one confluent T75) in 1.25ml of medium and seed onto each PGA scafffold. Make sure cell suspension has been uniformly dripped onto the PGA mesh-Dacron junction as well as onto the bottom side of PGA mesh.
- Wipe rim of bioreactor with alcohol wipe via rotating the bioreactor sideways avoid hovering.
- Silicone stopper lid assembly (Figure 2)
- Peel autoclave bag back carefully, making sure not to expose bottom of lid.
- Attach injection port to feeding tube at the male connector.
- Attach PTFE 0.20 μm filters to each of the three air ports.
- Be careful not to expose/touch bottom of lid during this process.
- Parafilm the injection port.
- Insert the silicone stopper lid into glass bioreactor and make sure that the feeding tube inside the bioreactor does not touch the seeded PGA scaffolds. Parafilm around the lid.
- Place bioreactor with the flow system inside the incubator (on its side) and rotate the bioreactor every 5 minutes for 25-30 minutes.
- Fill the bioreactor chamber with 400ml of our 4-10 culture media as described in (Table 1). This culture medium is "optimized" for porcine engineered arteries.
6. Day 6-7: Turning on the Pump, and First Feeding
- Grow the seeded scaffolds statically without any pulsatile pumping through the silicone tubing for 6-7 days. There is no need for medium change or vitamin C supplementation during this time.
- Make sure there are no leaks of PBS or kinking of flow system tubing before turning on the pump.
- Turn on the pump of the flow system and make sure to adjust pump setting so that the pressure reads approximately 270/-30mmHg.
- Record pressures daily throughout culture and maintain the pressure at 270/-30mmHg. Pressure transducer can be connected to a computer to read and monitor the pressure.
First Feeding
- Assemble injection port & PTFE filter onto feeding lids for both medium change and medium waste deposal purposes.
- Place the feeding tube firmly on the pump and insert one end to feeding port of the bioreactor and the other end to the feeding lid. Make sure to wipe the injection ports with ethanol wipes.
- Use a dual-directional Masterflex pump to pump out 200ml of medium. Then use a new feeding tube to pump 200ml of fresh medium back into the bioreactor. Always start with a very slow speed, especially when pumping medium back to the bioreactor.
- Change medium and supplement ascorbic acid 2x/week. To add ascorbic acid, use a 30ml syringe to take out 25ml of medium and place it aside in the tissue hood. Dissolve 25mg of ascorbic acid in 5ml of PBS and filter it through a 0.22 μm filter. First inject the sterile ascorbic acid into the feeding tube and add back the 25ml medium taken out earlier. The medium recipe is given in Table 1.
7. Representative Results:
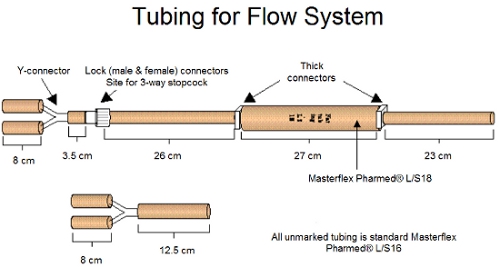
Figure 1. The tubing and connectors for the flow system assembly is shown above.
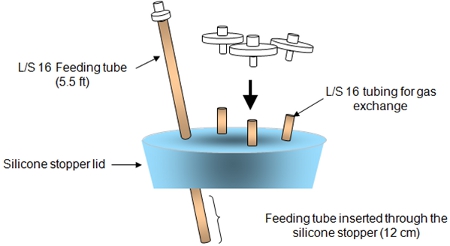
Figure 2. The silicone stopper lid assembly is shown above.

Figure 3. Schematics of bioreactor assembly are shown above. Inside the bioreactor Dacron cuffs are fastened onto the glass arms with the blue suture knots.

Figure 4. Flow system connected to tubing and bioreactor is shown above. L/S18 tubing will be pumped by a Masterflex pump and thus driving the flow. The pressure transducer will measure the pressure before entering the bioreactor at upper stream.

Figure 5. Image of harvested engineered vessel. Engineered vessels will appear to be opaque and achieve a wall thickness of approximately 250μm after 8-week culture under pulsatile conditions.
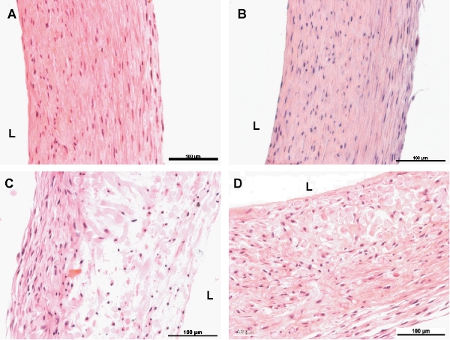
Figure 6. Haematoxylin and Eosin stained cross-sections of engineered vessels. A and B are 8-week non-pulsed and pulsed vessels, respectively. C and D are 4-week non-pulsed and pulsed vessels, respectively. L indicates the luminal side of the vessels. The scales bar is 100μm.

Figure 7. Masson’s Trichrome stains for collagen (blue) for cross-sections of engineered vessels. A and B are 8-week non-pulsed and pulsed vessels, respectively. C and D are 4-week non-pulsed and pulsed vessels, respectively. Note that the 4-week pulsed vessel shows more collagen than its non-pulsed counterpart. White arrows point to remaining PGA fragments in the vessels. The scales bar is 100μm.
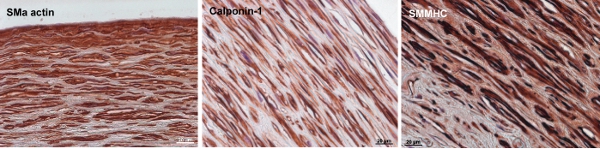
Figure 8. Immunochemistry staining of SMC markers in bovine engineered arteries. Smooth muscle α-actin, calponin-1, and smooth muscle myosin heavy chain (SMMHC) are early, intermediate, and late SMC contractile markers, respectively. By the end of 12-week culture, the cells in the vessel wall express SM α-actin and moderate amounts of Calponin-1 and SMMHC. The scales bar is 20μm.
| Component | Amount |
| DMEM(DME/low modified) | 500 ml |
| FBS (fetal bovine serum) heat inactivated | 100 ml |
| HEPES 1.0 M | 5ml |
| Vitamin C (dissolved in PBS or DMEM) | 25 mg |
| Proline/Glycine/Alanine 25 mg/25 mg/10 mg (dissolved in 5ml of PBS) | 5ml |
| CuSO4 1.5 μg (dissolved in1 ml of PBS) | 1ml |
| Penicillin G at 10,000 units/ml | 5ml |
| PDGF-BB (platelet-derived growth factor-BB) at 10ng/ml | 5μg |
| bFGF (basic fibroblast growth factor) at 10ng/ml | 5μg |
Table 1. Components of "4-10" medium are shown in the above table. With the exception of PDGF-BB and bFGF, all other components are to be filtered through a 0.2μm filter prior to use.
Discussion
The quality of engineered vessels is in large part dictated by the quality of the SMCs used in tissue culture. The critical aspects of SMC phenotype include contractile morphology, low passage number, and the ability to proliferate inside the bioreactor. We recommend that the passage number be no greater than P3 at the time of cell seeding onto the polymer scaffold. Moreover, it is crucial to confirm that the SMC sources are mycoplasma free prior to use. We have observed that mycoplasma-contaminated cells lead to substan...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work is funded by National Institutes of Health Grant R01 EB-008836 and R01 HL083895 (both to L.E.N.). We could like to thank Daryl Smith, the University Glassblower, for making the bioreactors for our research.
Materials
| Name | Company | Catalog Number | Comments |
| FBS (Fetal Bovine Serum) Heat-Inactivated | Hyclone | SH30071 | |
| DMEM | GIBCO, by Life Technologies | 11885 | |
| rhFGF-basic | R&D Systems | 234-FSE | |
| rrPDGF-BB | R&D Systems | 520-BB | |
| Penicilin G | Sigma-Aldrich | PENNA | |
| Copper(II) Sulfate | Sigma-Aldrich | C8027 | |
| Gylcine | Sigma-Aldrich | C8790 | |
| L-Alanine | Sigma-Aldrich | A7469-25G | |
| L-Proline | Sigma-Aldrich | P5607-25G | |
| Ascorbic Acid | Sigma-Aldrich | A4544-25G | |
| HEPES | Sigma-Aldrich | H3375-100G | |
| Silicone Stopper | Cole-Parmer | 06298-24 | |
| Masterflex tubes L/S | Cole-Parmer | 06508-16, 06508-18 | |
| Masterflex pump | Cole-Parmer | 7553-80 | |
| Dacron cuff | Maquet | 174406 | |
| PGA felt | Concordia | MO000877-01 | |
| 4-0 1.5 metric Surgipro II suture | Syneture | VP-557-X | |
| 6-0 0.7 metric Dexon suture | Syneture | 7538-11 | |
| 0.22μm PTFE filters | Whatman, GE Healthcare | 6780-2502 | |
| Three Way Stop-cock | Edwards Lifesciences | 593WSC | |
| Pressure Transducer | Edwards Lifesciences | PX212 | |
| IV bags | Baxter Internationl Inc. | R4R2110 | |
| Saline dilution set | Arrow International | W20030 | |
| Silicone tubing | Saint-Gobain | F05027 |
References
- Risau, W., Flamme, I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 11, 73-91 (1995).
- Fankhauser, F., Bebie, H., Kwasniewska, S. The Influcence of mechanical Forices and Flow Mechanisms on Vessel Occlusion. Lasers in Surgery and Medicine. 6, 530-532 (1987).
- Niklason, L. E., Gao, J., Abbott, W. M., Hirschi, K. K., Houser, S., Marini, R., Langer, R. Functional arteries grown in vitro. Science. 284, 489-493 (1999).
- Prabhakar, V., Grinstaff, M. W., Alarcon, J., Knors, C., Solan, A. K., Niklason, L. E. Engineering porcine arteries: Effects of scaffold modification. Journal of Biomedical Materials Research Part A. 67A, 303-311 (2003).
- Mitchell, S. L., Niklason, L. E. Requirements for growing tissue-engineered vascular grafts. Cardiovascular Pathology. 12, 59-64 (2003).
- Dahl, S. L. M., Rhim, C., Song, Y. C., Niklason, L. E. Mechanical properties and compositions of tissue engineered and native arteries. Annals of Biomedical Engineering. 35, 348-355 (2007).
- Quint, C., Kondo, Y., Manson, R. J., Lawson, J. H., Dardik, A., Niklason, L. E. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 108, 9214-9219 (2011).
- Niklason, L. E., Yeh, A. T., Calle, E. A., Bai, Y., Valentín, A., Humphrey, J. D. Enabling Tools for Engineering Collagenous Tissues Integrating Bioreactors, Intravital Imaging, and Biomechanical Modeling. Proceedings of the National Academy of Sciences of the United States of America. 107, 3335-3339 (2010).
- Gong, Z., Calkins, G., Cheng, E. -. c., Krause, D., Niklason, L. E. Influence of Culture Medium on Smooth Muscle Cell Differentiation from Human Bone Marrow-Derived Mesenchymal Stem Cells. Tissue Engineering Part A. 15, 319-330 (2009).
- Gong, Z. D., Niklason, L. E. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs. Faseb Journal. 22, 1635-1648 (2008).
- Poh, M. Blood vessels engineered from human cells. Lancet. 365, 2122-2124 (2005).
- American Heart Association. . Biostatistical fact sheet: cardiovascular procedures. , (2002).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved