A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Intraspinal Cell Transplantation for Targeting Cervical Ventral Horn in Amyotrophic Lateral Sclerosis and Traumatic Spinal Cord Injury
In This Article
Summary
Neural precursor transplantation is a promising strategy for protecting and/or replacing lost/dysfunctional cervical phrenic motor neurons in spinal cord injury (SCI) and the motor neuron disorder, amyotrophic laterals sclerosis (ALS). We provide a protocol for cell delivery to cervical spinal cord ventral horn in rodent models of ALS and SCI.
Abstract
Respiratory compromise due to phrenic motor neuron loss is a debilitating consequence of a large proportion of human traumatic spinal cord injury (SCI) cases 1 and is the ultimate cause of death in patients with the motor neuron disorder, amyotrophic laterals sclerosis (ALS) 2.
ALS is a devastating neurological disorder that is characterized by relatively rapid degeneration of upper and lower motor neurons. Patients ultimately succumb to the disease on average 2-5 years following diagnosis because of respiratory paralysis due to loss of phrenic motor neuron innnervation of the diaphragm 3. The vast majority of cases are sporadic, while 10% are of the familial form. Approximately twenty percent of familial cases are linked to various point mutations in the Cu/Zn superoxide dismutase 1 (SOD1) gene on chromosome 21 4. Transgenic mice 4,5 and rats 6 carrying mutant human SOD1 genes (G93A, G37R, G86R, G85R) have been generated, and, despite the existence of other animal models of motor neuron loss, are currently the most highly used models of the disease.
Spinal cord injury (SCI) is a heterogeneous set of conditions resulting from physical trauma to the spinal cord, with functional outcome varying according to the type, location and severity of the injury 7. Nevertheless, approximately half of human SCI cases affect cervical regions, resulting in debilitating respiratory dysfunction due to phrenic motor neuron loss and injury to descending bulbospinal respiratory axons 1. A number of animal models of SCI have been developed, with the most commonly used and clinically-relevant being the contusion 8.
Transplantation of various classes of neural precursor cells (NPCs) is a promising therapeutic strategy for treatment of traumatic CNS injuries and neurodegeneration, including ALS and SCI, because of the ability to replace lost or dysfunctional CNS cell types, provide neuroprotection, and deliver gene factors of interest 9.
Animal models of both ALS and SCI can model many clinically-relevant aspects of these diseases, including phrenic motor neuron loss and consequent respiratory compromise 10,11. In order to evaluate the efficacy of NPC-based strategies on respiratory function in these animal models of ALS and SCI, cellular interventions must be specifically directed to regions containing therapeutically relevant targets such as phrenic motor neurons. We provide a detailed protocol for multi-segmental, intraspinal transplantation of NPCs into the cervical spinal cord ventral gray matter of neurodegenerative models such as SOD1G93A mice and rats, as well as spinal cord injured rats and mice 11.
Protocol
Methods
1. Cell Preparation
As an example, we will describe the procedure for preparing glial progenitor cells 12 for transplantation because of our experience with this cell type. However, the specifics of the protocol, including medium and use of trypsin for example, will depend on the particular cell type being used for transplantation.
- Pre-warm all solutions to 37.0°C in water bath.
- Rinse flask 2X with HBSS. Add 5.0 mL/T-75 flask of 0.05% Trypsin/EDTA. Incubate flask for 3 minutes in 37.0°C incubator. Triturate 3X gently in flask with 5 or 10mL pipette.
Optional: Add 5.0 mL/T-75 flask of 1.0 mg/ml soybean trypsin inhibitor in DMEM/F12.
- Rinse each flask 2X in 5.0 mL of medium. Pool cells and rinses. Keep cells on ice for all subsequent steps.
- Spin at 200-300 g for 5 minutes in conical tube: preferably in centrifuge cooled to 4°C. Decant (and save) supernatant. Re-suspend cells in 1.0 mL of medium, and transfer to 1.5 mL Eppendorf tube.
- Count cells with hemocytometer using Trypan Blue to determine viability.
- Spin again in 1.5 mL tube (800 RPM for 10 minutes: preferably in centrifuge cooled to 4°C). Decant (and save) supernatant.
- Re-suspend cells in desired final volume of medium to achieve appropriate cell density.
- Distribute cell suspensions to multiple 1.5 mL Eppendorf tubes. Keep cells on wet ice until transplantation.
Do not use 0.75 mL tubes, as the Hamilton syringe/needle cannot fit deeply into this tube. Multiple injections will likely be made during the surgery sessions, and one wants to avoid disturbing the same tube of cells many times. Try to prepare at least 50% more volume of cell suspension than is needed. Keep cells on ice throughout the surgery session. Transplant cells within 4-5 hours of preparing cell suspensions in order to assure greatest viability of cells post-transplantation.
2. Preparation Prior to Surgery
- Conduct relevant baseline behavior assessments prior to surgery.
- Immune suppression: subcutaneous cyclosporine A (CSA: 10.0 mg/kg of body weight) or intraperitoneal (I.P.) FK-506/Rapamycin (1.0 mg/kg of body weight) should be started at least 3 days prior to transplantation, and should be given daily until sacrifice. Administration in the drinking water (instead of daily injections) is not advised. CSA is our immunosuppressant of choice when transplanting rodent-derived cells, while FK-506/Rapamycin is our choice when transplanting human-derived cells.
- Autoclave surgical tools prior to surgery. Prepare a neat and clean place to do surgery, as well as an uncluttered place to put tools. A glass-bead sterilizer is also useful. Keep all of tools clean (especially rongeur) during surgery, as this will make the procedure much easier. Any material used during the surgical procedure needs to be sterile.
- Instruments that cannot be sterilized such as the surgical microscope should be wiped down with an appropriate disinfectant and the handles covered with sterile material (e.g. gauze) so that the surgeon, once donned with sterile gloves, does not contaminate his/her gloves.
- The surgeon should wash her/his hands with a disinfectant (chlorhexidine scrub) before beginning surgery. The surgeon will wear sterile gloves, a facemask, and a clean gown.
3. Surgery: Animal Preparation and Surgery
Animal Prep:
- Weigh animal, and administer appropriate dose of anesthesia: Isofluorane inhalation or anesthetic cocktail (delivered via I.P.) of [acepromazine maleate (0.7 mg/kg), ketamine (95 mg/kg), and xylazine (10 mg/kg)].
- Pinch toe and/or use the palpebral reflex (touching the upper eyelid with cotton-tipped applicator to observe blinking) to determine if animal is properly anesthetized. The corneas should be protected by applying an artificial tear ointment prior to surgery.
- Shave back hair with electric razor (no attachment needed). Shave from slightly rostral to ears to the middle of the animal's back. Shave well, and remove cut hair from skin to prevent hair getting into incision. The surgical prep should be performed away from the area where the surgery will be performed to avoid contamination of the surgical site with stray hair.
- Soak gauze with Povidine-Iodine antiseptic solution, and apply to skin over shaved area. The surgical site should be scrubbed at least twice with the Povidine-Iodine solution, being careful to scrub from the center of the site toward the periphery. The site can then be rinsed with 70% alcohol or diluted germicidal scrub. The area should be covered with sterile drapes.
- Throughout the surgery, the use of a heating pad is highly recommended to maintain the animal's normal body temperature. In addition, body temperature should be monitored. Place animal on rolled gauze pad. For an adult rat, the rolled gauze pad should be ˜1.0 inch in thickness and ˜6.0 inches in length. Tape this pad to surgery board so that it does not move. Place animal on pad at level of chest/shoulder. Stretch out both arms on pad, and tape down arms to surgery board. The idea is to prop the cervical spinal cord up so that it is easier to work throughout the surgery since the cervical cord is found deep below the surface of the skin.
Surgery:
- On the lowest microscope magnification (we use 8 x magnification), use scalpel blade to make midline incision. Stretch skin laterally with other hand to make skin taut (which makes the skin easier to incise), and make incision from base of skull to scapula. (see Figure 1)
- Use scalpel blade to make incision through 3 muscle layers over the spinal column. Slightly squeeze the muscle (from lateral to medial) on both sides with other hand. Make sure to press firmly enough with scalpel to cut all muscle layers with the least number of cuts as possible so that muscle incisions will not be jagged, which makes suturing at end of surgery easier. However, do not press too firmly to prevent creating damage in deeper tissue (spinal column).
- With two cotton-tipped applicators, use twisting motion to separate overlying muscle from paraspinal muscle. One can be vigorous here. Do not cut or tear, as this will cause hemorrhage. Twisting motion with the applicators will nicely tease the tissue apart without causing damage or hemorrhage. If hemorrhage occurs at any stage of the surgery, it is best to be patient. Do not try to rush ahead with lots of hemorrhage because the blood will obscure vision of the surgical field. Cauterization or light pressure can be applied to the site of hemorrhage, but we find that patience is best. With practice, there should be little or no hemorrhage throughout this surgery.
- Retract muscle with 4 homemade retractors (see Figure 2). These retractors can be made from sturdy paperclips shaped into a retractor (see Figure 2-inset). Autoclave these before surgery. Care should be taken to blunt the ends of the retractors to avoid tissue damage. Tie string to retractor. Exposure should be square/rectangle-shaped. This shape can be achieved by pulling to 4 corners using the 4 retractors. Tape string to board in order to secure retractors (see Figure 2). Commercially-available retractors can also be used, but we prefer not to use these. It is crucial to create a good surgical field in order to clearly see throughout the surgery. Do not "blindly" continue along.
- Clear paraspinal muscle from dorsal surface of spinal column. To deal with paraspinal muscle on the dorsal surface of the spinal column, there are 2 potential strategies. For the first strategy, use rat-toothed forceps and medium-sized spring scissors to remove paraspinal muscle from dorsal surface of vertebrae. Try to make cuts very close to bone to better expose bone surface. Make cuts parallel to surface of vertebrae, but do not cut down into cord. For the second approach, make a midline incision in para-spinal muscle, and retract laterally with smaller retractors. In the second approach, the muscle can then be sutured back together after surgery.
- Clean surface of vertebral bone well. Use rongeur (the most important surgical instrument) to pull away muscle from levels C4, C5 and C6 (see Figure 3). This, of course, depends on the spinal levels that are targeted. While using rongeur with one hand, secure entire spinal column by grasping muscle over C2 process with rat-toothed forceps in other hand. C2 is the large rostral process in the surgical field. C3 is slightly under the muscle as well. C4, C5 and C6 are easy to access because they have little muscle overlying them.
- Start with laminectomy of C5. Again, secure by holding C2 with rat-toothed forceps. Grab entire lamina (see diagram: grab near midline) with rongeur. Position rongeur so that tool is completely perpendicular to axis of spinal column. Slowly crush lamina. Do not push down into spinal cord, as this will cause damage to spinal tissue. Crush and gently pull broken piece of bone upwards. Rongeur should crush piece so that one can easily pull away to remove. If piece of bone is still attached to the rest of the lamina, do not tug as this will cause hemorrhage and possible injury to spinal cord. Rongeur should be clean and sharp. Instruments such as the rongeur should be washed/wiped with sterile saline, which can be provided in a sterile bowl (see Figure 4).
For the initial opening of the laminectomy, positioning the rongeur perpendicular to the spinal column is preferable. This step can then be followed by taking small "nibbles" to extend the laminectomy by inserting the rongeur gently under the bone with an approximately 30-60 degree angle relative to the cord surface. - Extend laminectomy to all of C4-C6 laminae. Make one continuous opening in the bone over three spinal levels. The extent of the laminectomy can be adjusted based on the desired location(s) of intraspinal injection.
- Do not extend laminectomy too far laterally because this will cause hemorrhage. In order to target ventral horn, the injection site is relatively medial, so it is unnecessary to extend laminectomy far laterally. (see Figure 5)
- Increase magnification to ˜15 x.
- Clean off connective tissue (and possible dried blood) on top of dura with sharp / straight #5 forceps. The difference between connective tissue and dura needs to be learned with experience.
- Incise dura, a very tough meningeal layer, with either mini-spring scissors or microknife. Make incision parallel to axis of spinal column just medial to the entry zone of the dorsal rootlets. This will allow one to target the ventral horn. (see Figure 6)
- Use sharp / straight #5 forceps to grab and slightly lift dura off of spinal cord. Do not pinch / injure spinal cord. This takes some practice. Make incisions with microknife or very small spring scissors. Do not injure spinal cord.
- Extend incision slightly in rostral-caudal axis. Tension of dura will slightly separate dura to create a nice exposure of spinal cord.
- Make incisions at all appropriate sites for each intended injection. Dura has a hazy translucent appearance, while spinal cord surface has a brighter white-colored appearance. One will need to learn to distinguish these apart. It is advisable not to try to inject thru the dura with the injection needle. The dura is tough, so that it will be hard to pierce through it. In addition, piercing through the dura can affect the trajectory of the needle's insertion and will therefore affect the anatomically targeted delivery of cells.
- Inject cells bilaterally at 3 levels (6 sites total) to target large region of cervical enlargement. The number and location(s) of injections, of course, depends on the specific experiment.
- After piercing dura, cerebrospinal fluid will pour out. Dry surface of cord and entire exposure with gauze or cotton-tipped applicator.
- For cell injection, use 10.0μL Hamilton gastight RN syringe.
- Attach 33-gauge / 45-degree beveled metal RN Hamilton needle. The needle should be sharp. Use the same needle for only ˜ 20 injections (the needle will be sharper with less injections). One can also use pulled glass capillary tubes for needles. Use epoxy to attach pulled glass tip to 26-gauge blunt Hamilton metal needle. Trim outer tip diameter to ˜ 75.0-100.0 μL (depending on diameter of cells: one does not want to damage cells during the process of injection) using a surgical microscope and micrometer slide. We prefer the 33-gauge metal needles.
- Cells will fall out of suspension easily inside the Eppendorf tube on ice. Immediately prior to loading the injection syringe, gently tap tube until cells go back into suspension. Do not flick too intensely as this will injure cells and/or cause bubbles. Alternatively, use 20.0μL pipette-man to gently mix cells: pipette cell suspension only one or two times up and down.
- Load enough cell suspension volume for only one injection site. Cells will fall out of suspension inside injection syringe if one plans to do multiple injections. Slowly take up cells into syringe to avoid bubbles and/or damage to cells. Specifically for the glial progenitor cells that we work with in our lab, we inject 2.0 μL of cell suspension at a dilution of 50,000-75,000 cells per 1.0 μL over 2-5 minutes. We have found that 2uL/site does not result in tissue damage in the cervical spinal cord. Instead, our experience suggests that greatly increasing the numbers of cells in this volume (particularly if they are larger in size than our glial progenitors: 10.0 -20.0 μm in diameter) may cause damage to spinal cord parenchyma. We conducted an extensive cell dosing experiment to determine the optimal numbers of cells to inject at each site (at least in the intact cervical spinal cord). The parameters must be determined for the specific cell type and disease condition.
- Line up syringe/needle parallel with axis of spinal column to properly target desired anatomical region. The needle is angled just enough (approximately 80-degrees relative to the surgical table) to not bump the surgical scope head, but as close to 90-degrees as possible. Lower tip towards the spinal cord dorsal surface using microscope (see Figure 7).
- Aim needle just medial to entry zone of dorsal rootlets (see Figure 8). Gently touch surface of spinal cord with tip of needle. Slightly depress spinal cord with needle. Retract needle until spinal cord is back to normal. Record this position as the z = 0.0 using the ruler on the micromanipulator.
Note: We routinely do not stabilize the vertebral column during cervical spinal cord injections. Using our anesthetic regimen, up-and-down movement of the cervical spinal cord due to heavy breathing has never been an issue. However, if heavy breathing is a problem, the spinal column can be stabilized using a customized frame by gently clamping the bone and surrounding para-spinal muscle at levels C2 and C7 using modified forceps, being careful not to crush the vertebral bone or injure the underlying spinal cord tissue. If the animal's breathing becomes shallow during the procedure, more anesthetic drug should be provided. The surgery should be interrupted until a surgical plane of anesthesia has been re-achieved before resuming. - Slightly lower needle into spinal cord. Make sure to look in microscope while doing this. Lower needle to depth of 1.5 mm to target ventral horn in adult rats. Lower needle to depth of 0.75 mm to target ventral horn in adult mice. (see Figure 9). These depth numbers are representative of adult (at least 3 months old) female and male Sprague-Dawley rats (250-450 grams) and C57BL/6 mice (20-35 grams). Of course, depth and lateral position depend on the specific region of interest. Do not disturb injection system / surgery board or surgery table while needle is inserted into spinal cord to avoid damage to spinal tissue.
- Wait two minutes (longer is even better) after lowering needle to desired depth before injection.
- Inject 2.0 μL over 2-5 minutes at constant rate using pump controller.
- Wait two minutes (longer is better) after injection before slowly removing needle from spinal cord.
- Clean syringe with dH2O after each injection in order to prevent clogging. Slowly draw up and expel 3-5 times. Do not draw air into syringe.
- Return to lowest magnification (same as used at the beginning of surgery: ˜8 x). Suture dura closed using 9-0 suture, but this is definitely not necessary. It is also challenging. We sutured dura closed in the past, but we find no difference in animal behavior, transplant survival or spinal cord histology when the dura is not closed with suture. The dura can be covered with a protective piece of sterile gel foam. The gel foam should be placed directly on top of the dural incision site. However, it is important to take care in not abruptly pulling this piece of gelfoam off of the surface of the spinal cord during post-perfusion dissection, as the gelfoam can sometimes tightly adhere to the spinal cord surface.
- Optional: Suture paraspinal muscle closed with 4-0 suture.
- Suture closed three overlying muscle layers at one time with 4-0 suture. Suture muscles at three locations in the rostral-caudal axis (see Figure 10).
- Staple skin closed with 9.0 mm wound clips (see Figure 11). Tighten staples with needle holders to prevent animal from pulling off staples prior to full wound healing. Space staples approximately 0.5cm apart. Do not over-tighten the wound clips, as this can lead to impaired healing and necrosis.
- Apply Povidine-Iodine antiseptic solution to wound area with soaked gauze.
- Allow animal to recover on circulating warm water pad. Give sub-cutaneous injection of sterile Lactated Ringer's solution (5.0 mL for rat; 0.5 mL for mouse) immediately following surgery. Follow-up injections can also be provided if the animal appears dehydrated and/or listless. Prophylactic antibiotic treatment using 6.0mg/kg of Cefazolin can be used; however, our experience suggests that this transplantation procedure does not result in post-surgical infections if aseptic technique is followed and all instruments/surgical supplies are sterilized. If these precautions are followed, antibiotic administration is discouraged.
- Continue CSA or FK-506/Rapamycin daily until sacrifice.
- The entire surgery should take less than ˜ 45 minutes (with 6 injection sites). Otherwise, the animal will begin to wake up and breathe more heavily, which will adversely affect injection into spinal cord.
Troubleshooting Tips for Problems Associated with Various Steps of Protocol
Lack of/poor cell survival: This is likely not a technical issue associated with the injection, but is probably due to properties of the cell type being injected and/or to the immunesuppression regimen. These issues need to be empirically evaluated on a cell-type and animal model specific basis. The availability of a number of immune-deficient rat and mouse models are also available to circumvent problems with immunesuppression; however, these animals also present difficulties such as cost, the need for maintaining a colony, and additional caution necessary during surgery and housing.
Functional deficits observed following surgery: In our experience, we have not observed the occurrence of any functional deficits following this procedure when assessed by measures such as forelimb and hindlimb grip strength and phrenic nerve/diaphragm compounds muscle action potentials, even with 6 injections sites (of 2μL each) in the cervical spinal cord. Tissue destruction has also not been observed. Possible reasons for the occurrence of these problems include: not using the suggested needle gauge, injecting larger volumes and/or cell numbers, unintentional damage caused by improper laminectomy, lack of delicacy while cutting the dura (such as pinching the cord with the #5 forceps) or during the process of inserting the Hamilton needle into the spinal cord (replace a dull needle).
Cells not found in ventral gray matter: This could be due to a number of issues. If the injection misses the target medially or laterally, make sure to properly line up the injection system parallel with the axis of the spinal cord, and insert the needle just medial to the entry of the dorsal rootlets. If the injection misses the target dorsally or ventrally, make sure to zero your z-axis reading when the tip of the needle is just barely touching the dorsal surface of the spinal cord, use the suggested Hamilton needle with the appropriate bevel (a longer bevel can affect targeting), assure that the spinal cord does not compress when inserting the needle, take care in exactly measuring depth using the micromanipulator, and use animals within the suggested weight range. Adjust your technique accordingly if you are consistently injecting the cells in a location (which requires histological evaluation and subsequent technique modification). If the injections are randomly scattered at all of your injection sites, you will need to practice to improve consistency. If the cells tend to be located mostly at the dorsal aspect of the spinal cord along the needle track, wait longer before and after cell injection, and extend the actual injection over a longer period of time.
4. Representative Results:
Mouse-derived glial progenitor cells were transplanted (50,000 cells/site) into the ventral horn of spinal cord level C4 in an adult SOD1G93A rat. Mouse-derived transplanted cells can be distinguished from host rat tissue via detection with the mouse-specific antibody, M2. This image shows the survival of M2+ transplant-derived cells at 1 month post-transplantation (see Figure 12). The cells localized to the ventral gray matter, but the injection site medially missed the lateral ventral horn (the location of most phrenic motor neurons: denoted by dotted line). No cyst formation was seen when 50,000 cells were injected per site, and no behavioral impairment resulted from the injection procedure. However, injection of much higher numbers of cells (the actual number varies according to cell type, and should be systematically evaluated) results in damage at the injection site and along the needle track (see Figure 13: immunohistochemistry with the astrocyte marker, GFAP).
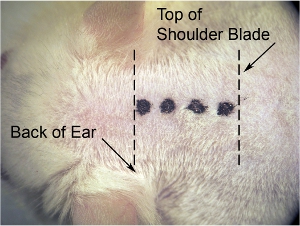
Figure 1. Initial incision of skin.On the lowest microscope magnification (we use 8 x magnification), use scalpel blade to make midline incision. Stretch skin laterally with other hand to make skin taut (which makes the skin easier to cut), and make incision (denoted by thick dotted line)from base of skull (at the level of the back of the ears) to shoulder blade (denoted by thin dotted line).
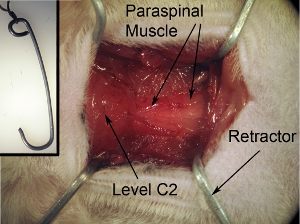
Figure 2. Exposure of surgical field.The surgical exposure should be square/rectangle-shaped. This shape can be achieved by pulling the surrounding muscle towards 4 corners using the 4 retractors. Tape string that is attached to retractors to surgical board in order to secure retractors and properly keep field open.
Figure 2-inset. Retractors for exposure of surgical field.The retractors are used to pull back muscle in order to create a surgical field with both clear visibility of the spinal cord and adequate space to perform surgery. Retractors can be made by shaping sturdy paperclips into the desired shape and size. Autoclave the retractors before for surgery. Tie string to retractor.
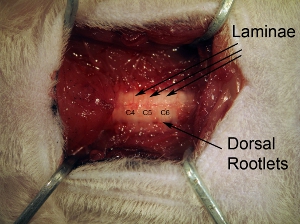
Figure 3. Vertebrae. Following removal of paraspinal muscle, thoroughly clean dorsal surface of vertebrae with rongeur. Individual lamina can be seen, as well as dorsal rootlets entering from lateral aspect of spinal column.
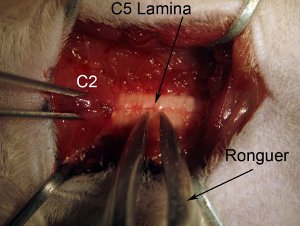
Figure 4. Laminectomy.Start laminectomy at spinal cord level C5. Secure spinal column by holding muscle overlying level C2 with rat toothed forceps. Grab entire lamina (see diagram: grab near midline) with rongeur. Position rongeur so that tool is completely perpendicular to axis of spinal column. Slowly crush lamina. Do not push down into spinal cord, as this will cause damage to spinal tissue. Crush and gently pull broken piece of bone upwards. Rongeur should crush piece so that one can easily pull away to remove. If piece of bone is still attached to rest of laminae, do not tug as this will cause hemorrhage and possible injury to spinal cord. Rongeur should be clean and sharp.
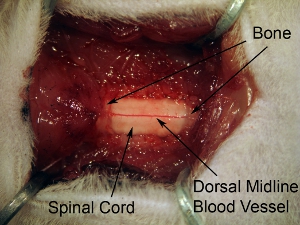
Figure 5. Exposure of spinal cord tissue following laminectomy.Extend laminectomy to expose all of C4-C6 spinal cord. Make 1 continuous opening in the bone over 3 spinal levels. Do not extend laminectomy too far laterally because this will cause hemorrhage. In order to target ventral horn, the injection site is relatively medial, so it is unnecessary to extend laminectomy to the complete lateral extent of the vertebral bone.
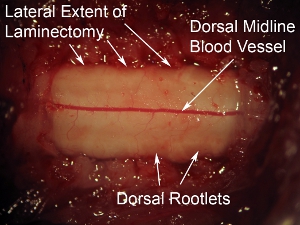
Figure 6. High magnification of dorsal surface of spinal cord.The prominent dorsal blood vessel can be seen running down the midline of the spinal cord. This blood vessel pattern is observed in most cases; however, some animals display a non-midline trajectory of the blood vessel. The dorsal rootlets can be seen at the lateral aspects of the spinal cord dorsal surface. Relative to the dura overlying the spinal cord, these nerves have a hazy appearance.
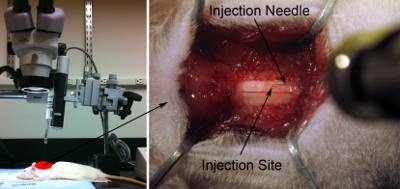
Figure 7. Injection setup.Line up syringe/needle parallel with axis of spinal column to properly target desired anatomical region. The needle is angled just enough (approximately 80-degrees relative to the surgical table) to not bump the surgical scope head, but as close to 90-degrees as possible (left panel).Lower injection tip towards the spinal cord dorsal surface using the microscope (right panel). Gently touch surface of spinal cord with tip of needle. Slightly depress cord with needle. Retract needle until spinal cord is back to normal flat state. Record this position as z = 0.0 using the ruler on the micromanipulator.
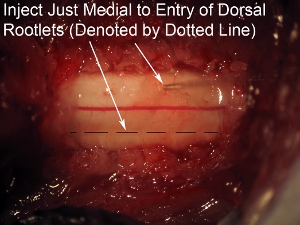
Figure 8. High magnification of spinal cord injection Aim needle just medial to the entry zone of the dorsal rootlets (denoted by dotted line).
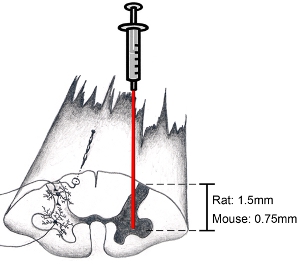
Figure 9. Diagram of spinal cord: how to target anatomical region of interest. When attempting to target the ventral horn, incise dura parallel to axis of spinal column just medial to the entry zone of the dorsal rootlets. This will allow one to target the ventral horn. Lower needle to depth of 1.5 mm to target ventral horn in adult rats (the age and sex of the animal does not make much of difference on depth). Lower needle to depth of 0.75 mm to target ventral horn in adult mice. Of course, depth and lateral position depend on the specific region of interest.
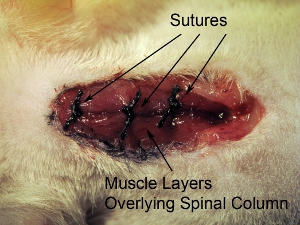
Figure 10. Closure of surgical site.Suture closed three overlying muscle layers at one time with 4-0 suture. Suture muscles at 3 locations in rostral-caudal axis.
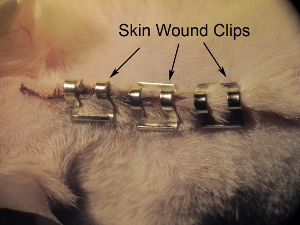
Figure 11. Stapling of skin.Staple skin closed with 9.0 mm wound clips. Tighten staples with needle holders to prevent the animal from pulling off staples prior to full wound healing. Space staples approximately 0.5 mm apart.
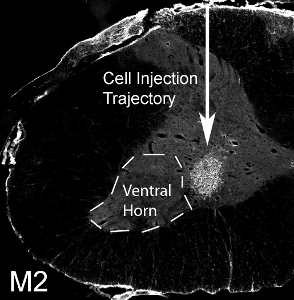
Figure 12. Transplantation of glial progenitors into cervical ventral horn. 50,000 mouse-derived glial progenitor cells were transplanted into the ventral horn of spinal cord level C4 in an SOD1G93A rat. M2+ mouse-derived transplanted cells survived at 1 month post-transplantation. The cells localized to the ventral gray matter, but the injection site medially missed the lateral ventral horn (denoted by dotted line).
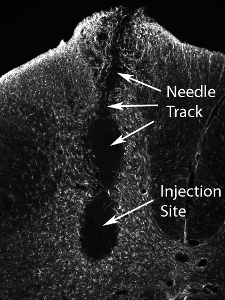
Figure 13. Tissue injury following transplantation of high numbers of neural precursor cells. Injection of much higher numbers of cells (the actual number varies according to cell type, and should be systematically evaluated) results in damage at the injection site and along the needle track.
Discussion
For studies involving SOD1G93A mice and rats, age- and sex-match the animals within a group, and distribute animals within the same litter to different groups. It is preferable to use all animals from the same sex for both ALS and SCI models because disease processes may differ between males and females; however, it may also be useful to have enough animals from both sexes to detect possible sex-specific effects, as this phenomenon has been reported with SOD1G93A rats 13 and SOD1G93A...
Disclosures
No conflicts of interest declared.
Acknowledgements
I would like to thank: all members of the Lepore, Maragakis and Rothstein labs for helpful discussion; The Paralyzed Veterans of America and the Craig H. Neilsen Foundation for funding.
Materials
| Name | Company | Catalog Number | Comments |
| HBSS | GIBCO, by Life Technologies | 14170 | |
| 0.05% Trypsin | GIBCO, by Life Technologies | 25300 | |
| Soybean Trypsin Inhibitor (optional) | Sigma-Aldrich | T-6522 | |
| Acepromazine maleate (0.7 mg/kg) | Fermenta Animal Health | ||
| Ketamine (95 mg/kg) | Fort Dodge Animal Health | ||
| Xylazine (10 mg/kg) | Bayer AG | ||
| #11 Feather surgical blade | Electron Microscopy Sciences | 72044-11 | |
| Cotton-tipped applicators (6 inch) | Fisher Scientific | 23-400-101 | |
| Rat-toothed forceps | Fine Science Tools | Rat: 11023-15; Mouse: 11042-08 | |
| Medium-sized spring scissors | Fine Science Tools | 15012-12 | |
| Mini spring scissors | Fine Science Tools | 15000-10 | |
| Rongeur | Fine Science Tools | Rat: 16121-14; Mouse: 16221-14 | |
| Microknife | Fine Science Tools | 10056-12 | |
| Needle holders | Fine Science Tools | 12502-14 | |
| Suture: 4-0 | Vicryl | S-183 | |
| Staples: 9 mm | Autoclip | 427631 | |
| Stapler: 9 mm (Reflex #203-1000) | World Precision Instruments, Inc. | 5000344 | |
| Warm water pump (T/Pump) | Gaymar Industries | P/N 07999-000 | |
| Cyclosporin A: 250.0 mg/5.0 mL ampules | Novartis AG | NDC 0078-0109-01 | |
| FK-506 | LC Laboratories | F-4900 | |
| Rapamycin | LC Laboratories | R-5000 | |
| Injector | World Precision Instruments, Inc. | UMP2 | |
| Micro 4 Microsyringe Pump Controller | World Precision Instruments, Inc. | UMC4 | |
| Micromanipulator | World Precision Instruments, Inc. | Kite-R | |
| 10.0 μL Hamilton syringe | Hamilton Co | 80030 | |
| Hamilton needles: 33-gauge, 45° bevel, 1 inch | Hamilton Co | 7803-05 | |
| Glass 20.0 μL microcapillary pipettes (optional) | Kimble Chase | 71900-20 |
References
- Lane, M. A., Fuller, D. D., White, T. E. Respiratory recovery following high cervical hemisection. Trends in neurosciences. 31, 538-538 (2008).
- Kaplan, L. M., Hollander, D. Respiratory dysfunction in amyotrophic lateral sclerosis. Clin. Chest. Med. 15, 675-675 (1994).
- Miller, R. G., Rosenberg, J. A., Gelinas, D. F. Practice parameter: the care of the patient with amyotrophic lateral sclerosis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology: ALS Practice Parameters Task Force. Neurology. 52, 1311-1323 (1999).
- Mitsumoto, H., Chad, D. A., Pioro, E. P., Davis, F. A. . Amyotrophic lateral sclerosis. , (1998).
- Tandan, R., Bradley, W. G. Amyotrophic leteral sclerosis: Part 2. Etiopathogenesis. Annals of neurology. 18, 419-419 (1985).
- Bruijn, L. I., Miller, T. M., Cleveland, D. W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 27, 723-723 (2004).
- Rosen, D. R., Siddique, T., Patterson, D. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 362, 59-59 (1993).
- Bruijn, L. I., Becher, M. W., Lee, M. K. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 18, 327-327 (1997).
- Gurney, M. E., Pu, H., Chiu, A. Y. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 264, 1772-1775 (1994).
- Howland, D. S., Liu, J., She, Y. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci. 99, 1604-1604 (2002).
- Nagai, M., Aoki, M., Miyoshi, I. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J. Neurosci. 21, 9246-9246 (2001).
- McDonald, W., Becker, D. Spinal cord injury: promising interventions and realistic goals. Am. J. Phys. Med. Rehabi. I82, S38-S38 (2003).
- Sandrow-Feinberg, H. R., Zhukareva, V., Santi, L. PEGylated interferon-beta modulates the acute inflammatory response and recovery when combined with forced exercise following cervical spinal contusion injury. Experimental neurology. 223, 439-451 (2010).
- Gage, F. H. Mammalian neural stem cells. Science. 287, 1433-1433 (2000).
- Lane, M. A., Lee, K. Z., Fuller, D. D. Spinal circuitry and respiratory recovery following spinal cord injury. Respiratory physiology & neurobiology. 169, 123-123 (2009).
- Lepore, A. C., Rauck, B., Dejea, C. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nature. 11, 1294-1294 (2008).
- Rao, M. S. Multipotent and Restricted Precursors in the Central Nervous System. Anat Rec. 257, 137-137 (1999).
- Rao, M. S., Mayer-Proschel, M. Glial- restricted precursors are derived from multipotent neuroepithelial stem cells. Dev Biol. 188, 48-48 (1997).
- Suzuki, M., Tork, C., Shelley, B. Sexual dimorphism in disease onset and progression of a rat model of ALS. Sexual dimorphism in disease onset and progression of a rat model of ALS. Amyotroph Lateral Scler. 8, 20-20 (2007).
- Lepore, A. C., Haenggeli, C., Gasmi, M. Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain research. 1185, 256-256 (2007).
- Veldink, J. H., Bar, P. R., Joosten, E. A. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 13, 737-737 (2003).
- Shumsky, J. S., Lepore, A. C. Transplantation of Neuronal and Glial Restricted Precursors into Contused Spinal Cord Improves Bladder and Motor Functions, Decreases Thermal Hypersensitivity, and Modifies Intraspinal Circuitry. J. Neurosci. 25, 9624-9624 (2005).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved