A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Production and Detection of Reactive Oxygen Species (ROS) in Cancers
In This Article
Summary
Here we propose simple methods to test and evaluate the presence of reactive oxygen species in cells.
Abstract
Reactive oxygen species include a number of molecules that damage DNA and RNA and oxidize proteins and lipids (lipid peroxydation). These reactive molecules contain an oxygen and include H2O2 (hydrogen peroxide), NO (nitric oxide), O2- (oxide anion), peroxynitrite (ONOO-), hydrochlorous acid (HOCl), and hydroxyl radical (OH-).
Oxidative species are produced not only under pathological situations (cancers, ischemic/reperfusion, neurologic and cardiovascular pathologies, infectious diseases, inflammatory diseases 1, autoimmune diseases 2, etc…) but also during physiological (non-pathological) situations such as cellular metabolism 3, 4. Indeed, ROS play important roles in many cellular signaling pathways (proliferation, cell activation 5, 6, migration 7 etc..). ROS can be detrimental (it is then referred to as "oxidative and nitrosative stress") when produced in high amounts in the intracellular compartments and cells generally respond to ROS by upregulating antioxidants such as superoxide dismutase (SOD) and catalase (CAT), glutathione peroxidase (GPx) and glutathione (GSH) that protects them by converting dangerous free radicals to harmless molecules (i.e. water). Vitamins C and E have also been described as ROS scavengers (antioxidants).
Free radicals are beneficial in low amounts 3. Macrophage and neutrophils-mediated immune responses involve the production and release of NO, which inhibits viruses, pathogens and tumor proliferation 8. NO also reacts with other ROS and thus, also has a role as a detoxifier (ROS scavenger). Finally NO acts on vessels to regulate blood flow which is important for the adaptation of muscle to prolonged exercise 9, 10. Several publications have also demonstrated that ROS are involved in insulin sensitivity 11, 12.
Numerous methods to evaluate ROS production are available. In this article we propose several simple, fast, and affordable assays; these assays have been validated by many publications and are routinely used to detect ROS or its effects in mammalian cells. While some of these assays detect multiple ROS, others detect only a single ROS.
Protocol
1. Detection of ROS using carboxy-H2DCFDA
Carboxy-H2DCFDA is non-fluorescent but in the presence of ROS, when this reagent is oxidized, it becomes green fluorescent.
- Immediately prior to use, prepare a fresh stock solution of carboxy-H2DCFDA in sterile dimethylsulfoxide (DMSO) or 100% ethanol. Avoid multiple thaw/freeze cycles of your dye.
- Wash the cells with HEPES buffered salt solution (HBSS) or phosphate-buffered saline (PBS) to remove traces of the original medium.
- Load the cells with the dye (and the control dye, cf note section). In this assay we used Jurkat, a human leukemia cell line. Use carboxy-H2DCFDA at a final concentration of 1μM in regular culture medium with reduced serum (2%).
- Incubate the cultures for 30 minutes in the dark, in a conventional incubator (37°C, 5% CO2). Discard all unused dye solutions.
- Remove carboxy-H2DCFDA containing medium and wash twice with HBSS or PBS. From this step forward, protect your cells from light.
- Add fresh medium containing your drug of choice to the carboxy-H2DCFDA -loaded cells and incubate as desired. For this example we use H2O2 (0.03%) for 1 hour.
- Assess ROS by immediately analyzing your cells by flow cytometry using the FL1 channel (green fluorescence), or by fluorescence plate reader, or by fluorescence microscopy. The fluorescence can be detected by using excitation and emission wavelengths that are appropriate for green fluorescence.
Note: Controls should include carboxy-H2DCFDA-loaded untreated cells and unstained untreated cells. Carboxy-H2DCFDA is known to detect peroxides but could also be oxidized by other ROS. This reagent can also be modified by other means, oxidation insensitive control dye such as 5-(and-6)-carboxy-2',7'-dichlorofluorescein diacetate (carboxy-DCFDA) should therefore be included in the test.
2. Measurement of nitric oxide (NO) production
You will need Sulfanilamide and N-1-napthylethylenediamine dihydrochloride (NED) solutions, and Nitrite standard. This assay is called the Griess assay.
NED solution : Make a 0.1% solution of N-1-napthylethylenediamine dihydrochloride diluted in sterile water.Sulfanilamide solution : Make a 1% solution of sulfanilamide diluted in 5% phosphoric acid. Nitrite Standard: Dilute the 0.1M standard stock sodium nitrite to 100μM in sterile medium, do a serial dilution in the same medium.
Storage Conditions: Store chemicals as directed by manufacturer at room temperature. When reconstituted, NED and Sulfanilamide solutions are stored immediately after use at 4°C, in the dark, and for a maximum of 3 months.
- Culture cells in a 96 well plate, use triplicates for each condition, and include proper controls according to your experiments.
- Treat cells to induce NO production. In our experiment we use lipopolysaccharide (LPS) (100 ng/ml) and recombinant IL-4 to treat our cells. In this protocol, we used RAW 264.7, a mouse macrophage cell line (Mouse leukaemic monocyte macrophage cell line).
- On the day of the assay, bring both reagents to room temperature.
- Spin your plate, collect cell supernatants and transfer 50μl to a new 96 well plate. Prepare limiting dilution wells of your standard stock solution to make a standard curve.
- Add 50μl of Sulfanilamide Solution to each sample and control well and mix well.
- Incubate at room temperature for 10 minutes in the dark.
- Add 50μl of N-1-napthylethylenediamine dihydrochloride solution to each sample and control well and mix well.
- Incubate at room temperature for 10 minutes in the dark.
- Measure absorbance immediately using a plate reader with filter of wavelengths between 520nm and 550nm.
If you utilize different plate/dish sizes, use 1/1/1 volume for each solution and sample supernatant.
A violet color will appear in the positive wells. Results obtained with the standard will help you check the stability of your solutions.
3. Detection of ROS action: oxidized proteins
A different method to identify the production of ROS is to look at the end results by detecting the oxidation of proteins. Indeed, ROS modify glutathione, an antioxidant that is expressed in most cells and plays a protective role against ROS. Following oxidation by ROS, modification of the reduced glutathione (GSH) results in the sulfhydryl (thiol) group of its cysteine being linked to a second glutathione via a disulfide bridge. This leads to the formation of a dimerized protein (oxidized protein GSSG). GSH can be restored via modification of GSSG by the enzyme glutathione reductase. The increase in the GSSG/GSH ratio reflects oxidative stress. The following assay is based on the detection and quantification of these oxidized proteins. This method is not selective for specific ROS but rather detects the effects of NO, H2O2, O2- and other ROS. Here we measure the total amount of oxidized (GSSG) and reduced (GSH) glutathione using bioluminescent signals.
- Seed your cells in a 96 well plate (white/transparent, flat bottoms) as usual. For our experiment, we used 1x104 cells per well for adherent Raw 264.7 and A549 cells and suspension Jurkat cells. Depending on the size of your cells these numbers could be adjusted. We recommend to plate adherent cells the day before the test to allow them to attach to the plate. Enough wells should be prepared for oxidized and reduced protein detection as well as for "medium only" and untreated cells as controls. Use triplicates for all conditions.
- Treat your cells with your drug for the required time. Here we treated Jurkat cells and A549 cells for 1h with H2O2 (at 5mM and 2.5mM respectively). Raw 264.7 cells were treated with 200ng/ml of LPS for 16 h. During this incubation time, bring all your reagents to room temperature (RT). Make all reagents no more than 30 minutes before performing the test. The table below shows the volumes of reagents per well. When possible, it is important to remove the media containing the reducing agent before proceeding with the test.
| Adherent cells | Suspension cells | |||
| Total Glu Lysis | Oxidized Glu Lysis | Total Glu Lysis | Oxidized Glu Lysis | |
| NEM, 25mM | none | 0.5μl | none | 0.5μl |
| Luciferin-NT | 1 μl | 1 μl | 1 μl | 1 μl |
| Passive Lysis Buffer, 5X | 10μl | 10μl | 10μl | 10μl |
| Distilled water | 39.0μl | 38.5μl | 14μl | 13.5μl |
| Final volume per well | 50μl | 50μl | 25μl | 25μl |
- Remove medium from wells with adherent cells. Do not remove medium in wells with suspension cells.
- Add reduced glutathione lysis reagent or oxidized glutathione lysis reagent to the corresponding wells and shake for 5 minutes at RT.
- Add the Luciferin generation reagent and incubate for 30 minutes at RT.
- Add the Luciferin detection reagent and incubate for 15 minutes at RT.
- Read the bioluminescent signal at integration time of 0.25-1 second per well using a plate reader luminometer.
| Adherent cells | Suspension cells | |
| Cell number per well | 1x104 | 1x104 |
| Cell suspension per well+ drug | 100 μl, to be removed before 3.4 | 25μl, not to be removed |
| Reduced Glutathione Lysis Reagent | 50 μl | 25μl |
| Oxidized Glutathione Lysis Reagent | 50 μl | 25μl |
| Luciferin Generation Reagent | 50 μl | 50 μl |
| Luciferin Detection Reagent | 100 μl | 100 μl |
4. Representative Results:
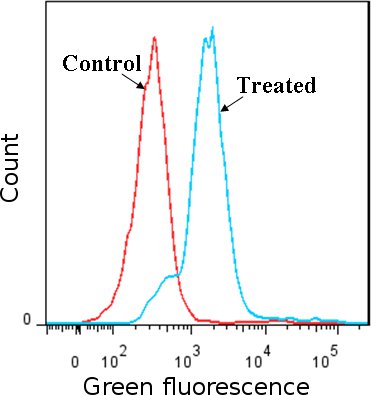
Figure 1 Detection of ROS using carboxy-H2DCFDA dye. Jurkat cells (human leukemia cell line) treated with H2O2 were compared to non-treated cells. ROS induces the modification of carboxy-H2DCFDA that fluoresces green as detected by flow cytometry, the fluorescent peak in H2O2 treated cells shift compared to the peaks in controls (H2O2 treated cells stained with oxidation insensitive dye and non-treated cells stained with carboxy-H2DCFDA) . Results confirm the presence of ROS in treated cells.

Figure 2 Detection of NO using Griess reagents. RAW 264.7 cells (mouse macrophage) were treated with LPS and IL-4. A significant increased in NO production was detected in treated cells compared to control untreated cells.
| Cell lines | Non-treated | Treated |
| Raw 264.7 | 13.0 | 8.3 |
| A549 | 21.6 | 10.5 |
| Jurkat | 5.2 | 2.8 |
Table 1 Detection of ROS mediated oxidation of proteins. RAW 264.7 cells were treated with LPS, Jurkat and A549 (human lung cancer) cells were treated with H2O2. Results are expressed as the ratio reduced (GSH)/oxidized (GSSG) glutathione. Lower ratios of glutathione (GSH)/(GSSG) were detected in treated cells compared to control untreated cells, revealing that proteins were more oxidized in treated cells.
Discussion
Several pathological situations such as inflammatory diseases, cancers, ischemia/reperfusion, and also treatments such as radiation or chemotherapy (i.e cisplatin) induce ROS overproduction. Thus, detecting and measuring ROS levels is important in many basic, pre-clinical and clinical studies. However, ROS have very short half lives and could be complicated to detect. Here, we propose simple tests that are routinely used and widely accepted for the detection of free radical production in mammalian cells.
Disclosures
We received the support of Promega for this publication.
Acknowledgements
This work was supported by National Institutes of Health (CA142664).
Materials
| Name | Company | Catalog Number | Comments |
| 5-(and-6)-carboxy-2’,7’-dichlorofluorescein diacetate (carboxy-DCFDA) | Molecular Probes, Life Technologies | C369 | control |
| carboxy-H2DCFDA | Molecular Probes, Life Technologies | C400 | |
| Sulfanilamide | Sigma-Aldrich | S9251-100G | |
| N-1-napthylethylenediamine dihydrochloride | Sigma-Aldrich | N9125-10G | |
| Nitrite standard | Sigma-Aldrich | 237213-100G | |
| GSH/GSSG-Glo Assay | Promega Corp. | V6612 | To quantify oxidized, reduced or oxidized/reduced glutathione |
References
- Guzik, T. J., Korbut, R., Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 54, 469-487 (2003).
- Perl, A., Gergely, P., Banki, K. Mitochondrial dysfunction in T cells of patients with systemic lupus erythematosus. Int Rev Immunol. 23, 293-313 (2004).
- Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T., Mazur, M., Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 39, 44-84 (2007).
- Droge, W. Free radicals in the physiological control of cell function. Physiol Rev. 82, 47-95 (2002).
- Nakamura, K., Yube, K., Miyatake, A., Cambier, J. C., Hirashima, M. Involvement of CD4 D3-D4 membrane proximal extracellular domain for the inhibitory effect of oxidative stress on activation-induced CD4 down-regulation and its possible role for T cell activation. Mol Immunol. 39, 909-921 (2003).
- Los, M., Droge, W., Stricker, K., Baeuerle, P. A., Schulze-Osthoff, K. Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur J Immunol. 25, 159-165 (1995).
- Deem, T. L., Cook-Mills, J. M. Vascular cell adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: role of reactive oxygen species. Blood. 104, 2385-2393 (2004).
- Pacher, P., Beckman, J. S., Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 87, 315-424 (2007).
- Griendling, K. K., Sorescu, D., Lassegue, B., Ushio-Fukai, M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 20, 2175-2183 (2000).
- Loh, K., Deng, H., Fukushima, A., Cai, X., Boivin, B., Galic, S., Bruce, C., Shields, B. J., Skiba, B., Ooms, L. M., Stepto, N., Wu, B., Mitchell, C. A., Tonks, N. K., Watt, M. J., Febbraio, M. A., Crack, P. J., Andrikopoulos, S., Tiganis, T. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 10, 260-272 (2009).
- Goldstein, B. J., Mahadev, K., Wu, X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 54, 311-321 (2005).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved