A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Formulation of Diblock Polymeric Nanoparticles through Nanoprecipitation Technique
In This Article
Summary
This article describes a nanoprecipitation method to synthesize polymer-based nanoparticles using diblock co-polymers. We will discuss the synthesis of diblock co-polymers, the nanoprecipitation technique, and potential applications.
Abstract
Nanotechnology is a relatively new branch of science that involves harnessing the unique properties of particles that are nanometers in scale (nanoparticles). Nanoparticles can be engineered in a precise fashion where their size, composition and surface chemistry can be carefully controlled. This enables unprecedented freedom to modify some of the fundamental properties of their cargo, such as solubility, diffusivity, biodistribution, release characteristics and immunogenicity. Since their inception, nanoparticles have been utilized in many areas of science and medicine, including drug delivery, imaging, and cell biology1-4. However, it has not been fully utilized outside of "nanotechnology laboratories" due to perceived technical barrier. In this article, we describe a simple method to synthesize a polymer based nanoparticle platform that has a wide range of potential applications.
The first step is to synthesize a diblock co-polymer that has both a hydrophobic domain and hydrophilic domain. Using PLGA and PEG as model polymers, we described a conjugation reaction using EDC/NHS chemistry5 (Fig 1). We also discuss the polymer purification process. The synthesized diblock co-polymer can self-assemble into nanoparticles in the nanoprecipitation process through hydrophobic-hydrophilic interactions.
The described polymer nanoparticle is very versatile. The hydrophobic core of the nanoparticle can be utilized to carry poorly soluble drugs for drug delivery experiments6. Furthermore, the nanoparticles can overcome the problem of toxic solvents for poorly soluble molecular biology reagents, such as wortmannin, which requires a solvent like DMSO. However, DMSO can be toxic to cells and interfere with the experiment. These poorly soluble drugs and reagents can be effectively delivered using polymer nanoparticles with minimal toxicity. Polymer nanoparticles can also be loaded with fluorescent dye and utilized for intracellular trafficking studies. Lastly, these polymer nanoparticles can be conjugated to targeting ligands through surface PEG. Such targeted nanoparticles can be utilized to label specific epitopes on or in cells7-10.
Protocol
1. Synthesis of PLGA-b-PEG polymer
- Poly(D,L-lactide-co-glycolide) (PLGA) with terminal carboxylate groups (PLGA-carboxylate) is dissolved in any solvent for PLGA (as mentioned in materials section) at a concentration of 5mM. PLGA can be dissolved at this concentration with gentle stirring.
- Both NHS (molecular weight 115.09) and EDC (molecular weight 191.7) are dissolved in the PLGA solution at a concentration of 25mM. (Both EDC and NHS are added in a stoichiometric excess of 5 times compared to PLGA). PLGA-carboxylate is converted into PLGA-NHS by adding EDC and NHS to PLGA-carboxylate solution with gentle stirring for about 1 hour.
- The reaction product PLGA-NHS is precipitated out by adding the washing solution methanol. Approximately 10 times volume excess of methanol is added to the solution. The solution is centrifuged at 2000 x g to precipitate out the PLGA-NHS and discard the supernatant (removes the traces of EDC and NHS. This procedure of washing with methanol is repeated at least three times.
- The PLGA-NHS pellet is dried under a vacuum for 30 minutes to remove any traces of the washing solution.
- The PLGA-NHS pellet is now re-dissolved in the same solvent at the same concentration that was used initially to dissolve PLGA. The heterobifunctional PEG (amine-PEG-carboxylate) is then added to the PLGA solution at a concentration of 5mM (stoichiometric ratio of 1:1). The mixture solution is incubated for 24 hours with constant stirring.
- After 24 hours, the reaction product PLGA-b-PEG block copolymer is precipitated out by adding the washing solution methanol in excess. Repeat the washing and centrifugation process as mentioned above three times. This will remove all excess unreacted PEG.
- The PLGA-b-PEG block copolymer is dried under vacuum.
2. PLGA-b-PEG nanoparticle preparation
Nanoparticles with PLGA core covered with PEG at the surface can be prepared with these diblock copolymers. A variety of different hydrophobic drugs can be encapsulated in such nanoparticles. Fluorescent compounds can be encapsulated in the nanoparticles or can be conjugated to PLGA and thus these nanoparticles can be used for fluorescence imaging.
Nanoprecipitation method is used to make nanoparticles especially when the desired cargo to be encapsulated is highly hydrophobic in nature.
- The PLGA-b-PEG block copolymer and the drug/cargo (to be encapsulated) are dissolved in any solvent which dissolves PLGA. PLGA can be dissolved by many common solvents, including acetonitrile, DCM, tetrahydrofuran, acetone or ethyl acetate. The choice of solvent is critical, as it influences the properties of nanoparticle. Hence, appropriate solvent should be used in this step.
- The polymer/drug mixture is then added dropwise to 3-5 volumes of stirring water giving a final polymer concentration of around 3 mg/mL. (Fig. 2)
- The stirring is continued for 2 hours under reduced pressure to allow the nanoparticles to form by self-assembly and remove traces of the organic solvent.
- Harvesting and purification: The nanoparticles are then concentrated by centrifugation at 2,700xg for 10 min using an Amicon filter (MWCO 20KDa), washed, and reconstituted in PBS. This removes all the un-entrapped drug/cargo. Basic biophysical characterizations, such as size, surface charge, and drug loading efficiency can be performed to better understand the properties of nanoparticles.
3. Storage
Freeze-drying is a commonly used method to store nanoparticles11. Freeze-drying will preserve the physical and chemical characteristics of the nanoparticles for long term stability12. The freeze drying process can cause stress on the particles and destabilize the formulation, so cryo-protectants (protection from freezing stress) and lyo-protectants (protection from drying stress) are commonly used. The choice of these protectants is determined by the desired length of storage time13.
- In freeze-drying, there is total solidification of the sample by freezing below its Tg.
- In the drying step, the ice is removed by sublimation. Temperature and pressure should be optimized to achieve an efficient freeze-drying process.
4. Representative results:
Characterization of PLGA-b-PEG Di-block Copolymer
Different techniques can be used to confirm the successful conjugation of polymers. The composition of PLGA-b-PEG can be characterized using a 400 MHz 1H nuclear magnetic resonance (NMR). Molecular weight of the formed product (PLGA-b-PEG) can be verified by Gel permeation chromatography (GPC). The PLGA-b- PEG molecular weight distribution curve and elution time should be different from PLGA and PEG alone. In combination, these techniques should characterize the formed product and determine whether the conjugation reaction was successful.
Characterization of PLGA-b-PEG nanoparticles
Particle size and size distribution can be measured by dynamic light scattering. Different parameters in the nanoprecipitation process affect the size of particles. Molecular weights of the polymers used initially (both PLGA and PEG) also effect the particle size distribution. Transition electron microscopy (TEM) can also be used to confirm the size distribution and structure of the nanoparticles as seen in figure 3. The particle size range is generally in the nm range. Large particle sizes with uneven size distribution could indicate either an error in the conjugation reaction or the nanoprecipitation method needs optimization. In addition, surface zeta potential can be measured by ZetaPALS.
The drug/cargo loading efficiency can be quantified with standard HPLC.
The particles are dissolved in organic solvent and HPLC can be performed to measure the absorbance of the drug/cargo (Fig. 4). The drug release kinetic study can be done where known fixed quantities of the nanoparticles are dialyzed in 30 Slide-A-Lyzer MINI Dialysis units. At fixed time intervals, the content in the dialysis unit is collected and equal volume of organic solvent is added to dissolve the nanoparticles. HPLC is done on these samples to quantify the drug/cargo content.
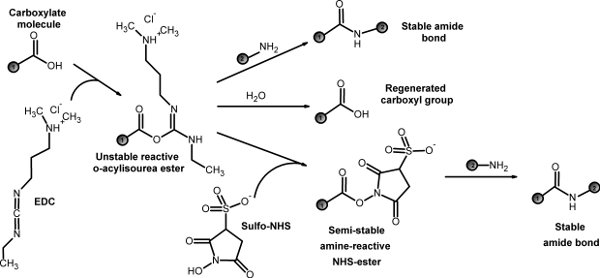
Figure 1. EDC/NHS chemistry
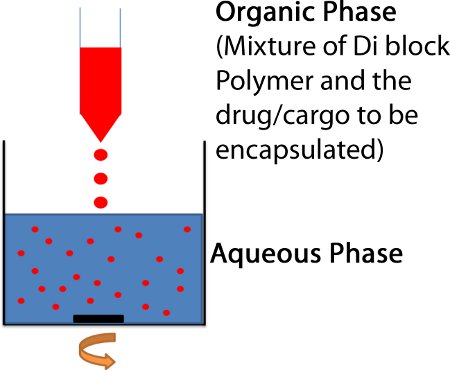
Figure 2. Nanoprecipitation method for preparing polymeric nanoparticles. The organic solution of a solvent (acetonitrile or DCM) containing the PEG-PLGA diblock and the drug or cargo to be loaded into the particle is added dropwise to 3-5 mL of stirring H2O.
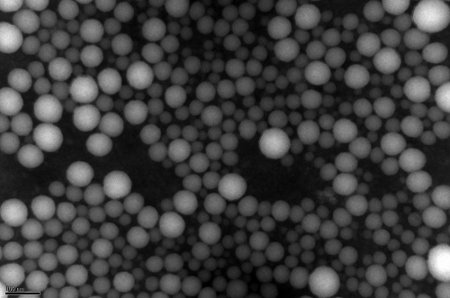
Figure 3. Transmission Electron Microscopy of nanopartices. A TEM image of PEG-PLGA nanoparticles containing wortamin. Phosphotungstic acid was used as a contrast agent.
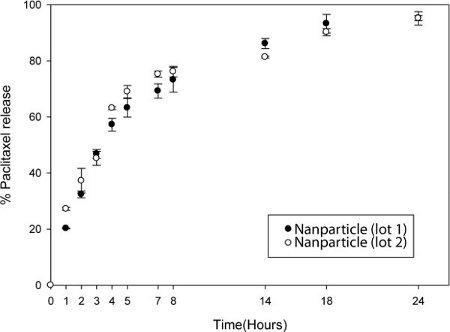
Figure 4. Controlled release of drug from nanoparticle. Release of Paclitaxel from nanoparticles after dialysis in PBS. At the noted time, particles were removed from dialysis cassettes and solublized in acetonitrile. The solution was measured by HPLC. Two separate lots of nanoparticles were compared.
Discussion
The nanoprecipitation method using diblock co-polymers represents a simple, fast method to engineer polymeric nanoparticles. The resulting nanoparticles are composed of a hydrophobic core which can be utilized for the delivery of poorly soluble compounds. The surface hydrophilic layer enables excellent aqueous solubility while providing a moiety for potential further conjugation to a targeting ligand.
There are many nanoparticle platforms, including liposomes, polymeric nanoparticles, dendrime...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was funded by the Golfers Against Cancer, Carolina Center for Nanotechnology Excellence Pilot grant, University Cancer Research Fund and National Health Institute K-12 Career Development Award.
Materials
| Name | Company | Catalog Number | Comments |
| EDC | Thermo Fisher Scientific, Inc. | 22980 | Conjugation Reagent |
| NHS | Thermo Fisher Scientific, Inc. | 24500 | Conjugation Reagent |
| amine-PEG-carboxylate | Laysan Bio Inc. | Nh2-PEG-CM-5000 | Polymer (Can use any PEG MW, 5000 is listed here) |
| PLGA-carbxylate |  Lactel Lactel | B6013-2 | Polymer |
| Dichloromethane (DCM) | Sigma-Aldrich | 34856 | Solvent |
| Acetonitrile >99% purity | Sigma-Aldrich | 34851 | Solvent |
| Methanol >99% purity | Sigma-Aldrich | 34860 | Wash |
References
- Drotleffa, S., Lungwitz, U., Breuniga, M., Dennis, A., Blunk, T., Tessmarc, J., Goëpferich, A. Biomimetic polymers in pharmaceutical and biomedical sciences. European Journal of Pharmaceutics and Biopharmaceutics. 58, 385-407 (2004).
- Bulte, J. W. M. . Nanoparticles in Biomedical Imaging. 3, (2008).
- Omid, C., Farokhzad, R. L. Impact of Nanotechnology on Drug Delivery. ACS NANO. 3, 16-20 (2009).
- Li, Y. -. P., Pei, Y. -. Y., Xian-Ying, Z., Zhou-Hui, G., Zhao-Hui, Z., Wei-Fang, Y., Jian-Jun, Z., Jian-Hua, Z., Xiu-Jian, G. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. Journal of Controlled Release. 71, 203-211 (2011).
- Hermanson, G. T. . Bioconjugate techniques. , (2008).
- Jeong, B., Bae, Y. H., Lee, D. S., Kim, S. W. Biodegradable block copolymers as injectable drug-delivery systems. Nature. 388, 860-862 (1997).
- Yoo, H. S., Park, T. G. Folate receptor targeted biodegradable polymeric doxorubicin micelles. Journal of Controlled Release. 96, 273-283 (2004).
- Cheng, J., Teply, B. A., Sherifi, I., Sung, J., Luther, G., Gu, F. X., Levy-Nissenbaum, E., Radovic-Moreno, A. F., Langer, R., Farokhzad, O. C. Formulation of Functionalized PLGA-PEG Nanoparticles for In Vivo Targeted Drug Delivery. Biomaterials. 28, 869-876 (2007).
- Gu, F., Zhang, L. F., Teply, B. A., Mann, N., Wang, A., Radovic-Moreno, A. F., Langer, R., Farokhzad, O. C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proceedings of the National Academy of Science. 105, 2586-2591 (2008).
- Sanna, V., Pintus, G., Roggio, A. M., Punzoni, A., Posadino, A. M., Arca, A., Marceddu, S., Bandiera, P., Uzzau, S., Sechi, M. Targeted Biocompatible Nanoparticles for the Delivery of (-)-Epigallocatechin 3-Gallate to Prostate Cancer Cells. J. Med. Chem. 54, 1321-1332 (2011).
- Abdelwahed, W., Degobert, G., Stainmesse, S., Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Advanced Drug Delivery Reviews. 58, 1688-1713 (2006).
- Holzer, M., Vogel, V., Mäntele, W., Schwartz, D., Haase, W., Langer, K. Physico-chemical characterisation of PLGA nanoparticles after freeze-drying and storage. European Journal of Pharmaceutics and Biopharmaceutics. 72, 428-437 (2009).
- Lee, M. K., Kim, M. Y., Kim, S., Lee, J. Cryoprotectants for Freeze Drying of Drug Nano-Suspensions: Effect of Freezing Rate. Journal of Pharmaceutical Sciences. 98, 4808-4817 (2009).
- Wang, A. Z. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert opinion on biological therapy. 8, 1063-1070 (2008).
- Jeong, B., Bae, Y. H., Kim, S. W. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J. Control Release. 63, 155-163 (2000).
- Gref, R. Biodegradable long-circulating polymeric nanospheres. Science. 263, 1600-1603 (1994).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved