A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Isolation of Mouse Lung Dendritic Cells
In This Article
Summary
A highly purified preparation of mouse lung dendritic cells is described. Specific emphasis is given to the isolation of conventional dendritic cell subset.
Abstract
Lung dendritic cells (DC) play a fundamental role in sensing invading pathogens 1,2 as well as in the control of tolerogenic responses 3 in the respiratory tract. At least three main subsets of lung dendritic cells have been described in mice: conventional DC (cDC) 4, plasmacytoid DC (pDC) 5 and the IFN-producing killer DC (IKDC) 6,7. The cDC subset is the most prominent DC subset in the lung 8.
The common marker known to identify DC subsets is CD11c, a type I transmembrane integrin (β2) that is also expressed on monocytes, macrophages, neutrophils and some B cells 9. In some tissues, using CD11c as a marker to identify mouse DC is valid, as in spleen, where most CD11c+ cells represent the cDC subset which expresses high levels of the major histocompatibility complex class II (MHC-II). However, the lung is a more heterogeneous tissue where beside DC subsets, there is a high percentage of a distinct cell population that expresses high levels of CD11c bout low levels of MHC-II. Based on its characterization and mostly on its expression of F4/80, an splenic macrophage marker, the CD11chiMHC-IIlo lung cell population has been identified as pulmonary macrophages 10 and more recently, as a potential DC precursor 11.
In contrast to mouse pDC, the study of the specific role of cDC in the pulmonary immune response has been limited due to the lack of a specific marker that could help in the isolation of these cells. Therefore, in this work, we describe a procedure to isolate highly purified mouse lung cDC. The isolation of pulmonary DC subsets represents a very useful tool to gain insights into the function of these cells in response to respiratory pathogens as well as environmental factors that can trigger the host immune response in the lung.
Protocol
1. Lung perfusion and single cell suspension
- Euthanize the mouse with an intraperitoneal injection of ketamine/xylazine anesthetic mixture (in mg/mouse ketamine 1.8, xylazine 0.19) and exsanguination via the femoral vein
- Expose the thoracic cavity by cutting and gently pulling back the outer skin of the peritoneum. Proceed to open the diaphragm by cutting the rib cage to expose both the heart and lungs.
- Perfuse gently the lungs using a 5 ml syringe filled with EDTA-HBSS (1mM EDTA in calcium/Magnesium-free HBSS). Connect a 25 G needle and insert needle into the right ventricle. To prevent leakage during injection, secure the myocardiac tissue around the needle by using forceps. Gently inject solution while maintaining a constant pressure. Accurate perfusion will result in lung inflation and a color change to pink/white.
- Remove draining lymph nodes to discard any potential contamination from this tissue.
- Collect lung tissue, transfer to a petri dish on ice, and chop it to small pieces using a razor blade.
- Transfer grounded tissue into a gentleMACS C tube containing 5 ml/lung of collagenase digestion solution (collagenase type 1A 0.5 mg/ml plus type IV bovine pancreatic DNase 20 μg/ml in HBSS containing 5% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin).
- Use the gentleMACS dissociator to obtain a single cell suspension. Choose program: m_lung_01. Incubate sample at 37 °C for 30 minutes. Shake tube every 5 minutes to resuspend tissue fragments. Proceed with program m_lung_02.
- Transfer sample to a 15 ml conical tube and centrifuge for 10 minutes at 335 x g at 4 °C. After this step, keep all reagents and centrifugations at 4 °C to prevent reduction in cell viability.
- Discard supernatant and lyse remaining red blood cells by adding 2 ml of ACK lysing buffer for 1 minute at room temperature. Wash cells with 13 ml of cold PBS/0.5% BSA and centrifuge for 10 minutes at 335 x g at 4°C.
- Discard supernatant, resuspend total cells in 5 ml of cold PBS/0.5%BSA and pass thorough a 100 μm nylon mesh.
- Determine the total cell number by using trypan blue exclusion dye.
2. Magnetic isolation and CD11c+ cells enrichment
- Centrifuge single cell suspension for 10 minutes at 200 x g at 4 °C and discard supernatant.
- Resuspend cell pellet in 400 μl of separation buffer (PBS, 0.5% BSA, and 2 mM EDTA) per 108 total cells. Block Fc-mediated unspecific antibody binding by using anti-CD16/CD32 antibody (0.5μg/ 106 cells) for 30 minutes at 4 °C.
- Wash cells with 10 ml of cold separation buffer and centrifuge for 10 minutes at 200 x g at 4 °C. Resuspend cells in 400 μl of separation buffer.
- Add 100 μl of CD11c MicroBeads per 108 total cells. Mix well and incubate for 15 minutes in the refrigerator (2-8 °C).
- Wash cells with 10 ml of cold separation buffer and centrifuge for 10 minutes at 200 x g at 4 °C and discard supernatant.
- Resuspend the cell pellet in 3 ml of cold separation buffer.
- Perform magnetic separation using the autoMACS ProTM Separator by selecting the program posseld. Collect the positive fraction (0.5 ml).
3. Conventional dendritic cell (cDC) isolation
- Incubate enriched CD11c positive cell suspension with anti-CD11c PE-Cy7 and anti-I-A/I-E (MHC-II)-FITC antibodies for 30 minutes at 4 °C. Optimized concentration of antibodies is 0.5 μg/ 106 of cells.
- Wash cells with cold PBS/0.5% BSA and centrifuge for 10 minutes at 335 x g at 4 °C. Resuspend cells in 0.5 ml of PBS/0.5% BSA and pass them through a 40 μm nylon mesh.
- Proceed to sort the cells by using the FACS Aria cell sorter in the purity mode. Add 100 μl of PBS/0.5% BSA to the collection tubes in order to prevent damage of purified cells. Keep your cells at a refrigerated temperature throughout the cell sorting process.
4. Alternate protocol for obtaining a single cell suspension and CD11c+ cells enrichment
- In replacement of the gentleMACS dissociator, grounded lung tissue from step 1.5 can be transferred to a 15 ml conical tube containing 5 ml of collagenase solution per lung and incubate for 1 hour at 37 °C. Vortex cells every 15 minutes in order to resuspend tissue fragments. Disrupt the tissue by passing the sample 6-8 times in and out through a 3 ml syringe connected to a 20 G needle. Avoid making bubbles during the process, otherwise the cell viability will be compromised. Once single cell suspension is obtained, continue to step 1.8.
- In replacement of the AutoMACSProTM separator, magnetic isolation for the enrichment of the CD11c cells, can also be performed by passing the cell suspension manually through two MACS columns. The selection of the appropriate columns will vary depending of the sample size.
5. Representative Results:
Lung cDC are identified as CD11chi/MHC-IIhi cell population. As shown in Fig. 1, cDC represent a smaller percentage of 1.4% when compared to some other tissues such as spleen (4.8%). However, in contrast to spleen, lung CD11c positive cells express different amounts of MHC-II, including a cell population different than that of cDC. After the single-cell preparation, the cell yield of total lung cells was about 3.0 to 3.8 x 107 cells/lung with a viability of 60-70 % from which a little over 1% represented cDC subset. This percentage was increased after the CD11c magnetic isolation where the total lung cDC was incremented about 10 times (>16%) from the original preparation (Fig. 2A). However, CD11c cells expressing low amounts of MHC-II (CD11chi/MHC-IIlo, macrophages) represented a high contaminating cell population (>70%) which was mixed with the lung cDC subset. As shown in Fig. 2B, those cells were eliminated after the cell sorting step when the cDC purity reached >96%. The usual yield of cDC after the whole procedure was 5 x 104 cells/lung. Microphotographs show morphological characteristics of cDC as rounded cells with the typical dendrites (upper panel). On the other hand, CD11chi/MHC-IIlo represented a morphologically different subset that shows cellular protuberances which are typical of macrophages (MΦ, lower panel) 12.
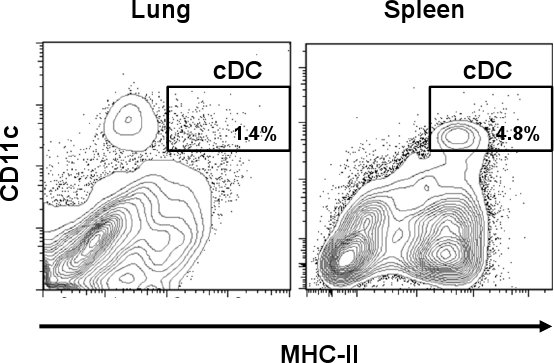
Figure 1. Differential DC frequency in mouse lung and spleen. Lung and spleen tissue from C57BL/6 mice were collected and treated with collagenase. Following collagenase digestion, cells were stained with anti-mouse CD11c-PE-Cy7 and anti-mouse I-A/I-E-FITC. Representative flow cytometry plots show percentages of cDC (CD11chi/MHC-IIhi) in lung and spleen.
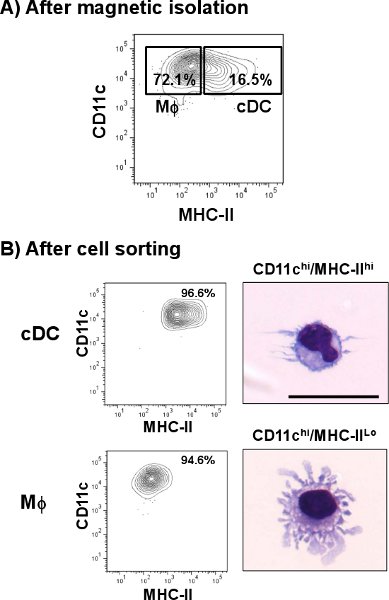
Figure 2. Isolation of cDC from mouse lung. Mouse lung single cell suspension was labeled with anti-CD11c microbeads and passed through an automatic cell separator. A) A representative plot shows the percentages of cDC (CD11chi/MHC-IIhi) and Macrophages (MΦ, CD11chi/MHC-IIlo) populations after CD11c enrichment. Further staining of the enriched fraction followed using anti-mouse CD11c-PE-Cy7 and anti-mouse I-A/I-E-FITC. Double positive cells were sorted and analyzed by flow cytometry. To identify their morphology, cells were cytospun and stained with a modified Wright-Giemsa staining. B) Representative plots show percentages of cDC and MΦ after cell sorting. Microphotographs show a representative image of lung cDC and lung MΦ. Scale bar = 20 μm.
Discussion
Isolation of pulmonary mouse DC is an important technique for the study of a wide range of respiratory stimuli. The process of obtaining these cells includes critical steps that prevent loss of cells as well as cell viability and purity. Perfusing the lung before collection will help to eliminate any peripheral cells as well as reduce contaminant erythrocytes. The use of the automated dissociation can be advanteogus when a large number of lungs are handled, otherwise you can opt for the alternative protocol, keeping in m...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank to Marilyn Dietrich at the LSU Flow Cytometry Core Facility for her help with the cell sorting and Peter Mottram for his assistance with the microphotographs. This work was funded by the Flight Attendant Medical Research Institute, the LSU-Competitive Research Program Award, and the NIH/NIAID Grants P20 RR020159 and R03AI081171.
Materials
| Name | Company | Catalog Number | Comments |
| ACK lysing buffer | Invitrogen | D6-0005DG | |
| Anti-mouse CD11c (HL3) | BD Biosciences | 5580979 | PE-Cy7 conjugated |
| Anti-mouse I-A/I-E (269) | BD Biosciences | 553623 | FITC conjugated |
| Collangenase Type 1A | Sigma-Aldrich | 9891-500MG | |
| Cell strainers | BD Biosciences | 352340, 352360 | |
| CD11c (N418) Microbeads | Miltenyi Biotec | 130-052-001 | |
| DNase I | Sigma-Aldrich | D5025-150KU | |
| Hank’s Balanced Salt solution | Invitrogen | 14170 | |
| Hepes buffer solution | Invitrogen | 15630 | |
| Petri dishes 60 mm | BD Biosciences | 351016 | |
| GentleMACS™ C tubes | Miltenyi Biotec | 130-093-237 | |
| Gentle MACS dissociator | Miltenyi Biotec | 130-093-235 | |
| AutoMACS-Pro™ | Miltenyi Biotec | 130-092-545 | |
| FASCS Aria | BD Biosciences |
References
- Pulendran, B., Palucka, K., Banchereau, J. Sensing pathogens and tuning immune responses. Science. 293, 253-256 (2001).
- Banchereau, J. Immunobiology of dendritic cells. Annual Review of Immunology. 18, 767-811 (2000).
- Manicassamy, S., Pulendran, B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 241, 206-227 (2011).
- Steinman, R. M., Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 137, 1142-1162 (1973).
- Asselin-Paturel, C. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2, 1144-1150 (2001).
- Chan, C. W. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat. Med. 12, 207-213 (2006).
- Taieb, J. A novel dendritic cell subset involved in tumor immunosurveillance. Nat. Med. 12, 214-219 (2006).
- Guerrero-Plata, A., Kolli, D., Hong, C., Casola, A., Garofalo, R. P. Subversion of pulmonary dendritic cell function by paramyxovirus infections. Journal of Immunology. 182, 3072-3083 (2009).
- Larson, R. S., Springer, T. A. Structure and function of leukocyte integrins. Immunol. Rev. 114, 181-217 (1990).
- Sung, S. S. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 176, 2161-2172 (2006).
- Wang, H. Local CD11c+ MHC class II- precursors generate lung dendritic cells during respiratory viral infection, but are depleted in the process. J. Immunol. 177, 2536-2542 (2006).
- Bhatia, S. Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype. PLoS One. 6, e15943-e15943 (2011).
- Shao, Z., Makinde, T. O., McGee, H. S., Wang, X., Agrawal, D. K. Fms-like tyrosine kinase 3 ligand regulates migratory pattern and antigen uptake of lung dendritic cell subsets in a murine model of allergic airway inflammation. J. Immunol. 183, 7531-75381 (2009).
- Hao, X., Kim, T. S., Braciale, T. J. Differential response of respiratory dendritic cell subsets to influenza virus infection. J. Virol. 82, 4908-4919 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved