Generation of an Immortalized Murine Brain Microvascular Endothelial Cell Line as an In Vitro Blood Brain Barrier Model
In This Article
Summary
This method describes how to isolate and immortalize microvascular endothelial cells from mouse brain. We describe a step-by-step protocol starting from the homogenization of brain tissue, digestion steps, seeding and immortalization of the cells. Usually, it takes about five weeks to obtain a homogenous, immortalized microvascular endothelial cell line.
Abstract
Epithelial and endothelial cells (EC) are building paracellular barriers which protect the tissue from the external and internal environment. The blood-brain barrier (BBB) consisting of EC, astrocyte end-feet, pericytes and the basal membrane is responsible for the protection and homeostasis of the brain parenchyma. In vitro BBB models are common tools to study the structure and function of the BBB at the cellular level. A considerable number of different in vitro BBB models have been established for research in different laboratories to date. Usually, the cells are obtained from bovine, porcine, rat or mouse brain tissue (discussed in detail in the review by Wilhelm et al. 1). Human tissue samples are available only in a restricted number of laboratories or companies 2,3. While primary cell preparations are time consuming and the EC cultures can differ from batch to batch, the establishment of immortalized EC lines is the focus of scientific interest.
Here, we present a method for establishing an immortalized brain microvascular EC line from neonatal mouse brain. We describe the procedure step-by-step listing the reagents and solutions used. The method established by our lab allows the isolation of a homogenous immortalized endothelial cell line within four to five weeks. The brain microvascular endothelial cell lines termed cEND 4 (from cerebral cortex) and cerebEND 5 (from cerebellar cortex), were isolated according to this procedure in the Förster laboratory and have been effectively used for explanation of different physiological and pathological processes at the BBB. Using cEND and cerebEND we have demonstrated that these cells respond to glucocorticoid- 4,6-9 and estrogen-treatment 10 as well as to pro-infammatory mediators, such as TNFalpha 5,8. Moreover, we have studied the pathology of multiple sclerosis 11 and hypoxia 12,13 on the EC-level. The cEND and cerebEND lines can be considered as a good tool for studying the structure and function of the BBB, cellular responses of ECs to different stimuli or interaction of the EC with lymphocytes or cancer cells.
Protocol
1. Isolation of Brain Microvessels
- For each preparation use five to ten neonatal mice of either sex (3-5 days old).
- Euthanize a mouse according to local IACUC/veterinarian recommendations, remove the brain immediately, and transfer into a Petri dish containing the following solution: 15 mM Hepes (pH 7.4), 153 mM NaCl, 5.6 mM KCl, 2.3 mM CaCl2x 2H2O, 2.6 mM MgCl2x 6H2O, 1% (w/v) BSA (hereafter referred to as buffer A).
- Unless otherwise indicated, all isolation procedures should be performed at room temperature (RT) (22-24 °C) under the laminar flow hood. Cut the brains (cerebrum without cerebellum and brain stem), after removal of the meninges and capillary fragments, in buffer A using a sterile scalpel. Pipette the tissue fragments up and down using a 10 ml pipette until no clumps appear. Transfer the suspension into a 50 ml Falcon tube and centrifuge at 250 x g for 5 min at RT.
Note: Meninges can be identified as thin, transparent membranes at the surface of the brain tissue. They can be carefully removed with sterile forceps.
- Discard the supernatant.
- Dissolve the pellet in 4.5 ml Buffer A with 1.5 ml of 0.75% (w/v) collagenase/ dispase (Roche) and incubate for 45 min at 37 °C in a water bath (occasionally shaking).
- Meanwhile, prepare the 12-well plates by coating four wells with collagen IV (0.1 mg/ml dissolved in 50 mM acetic acid). Allow to adhere for 1 hr.
- Stop the digestion by addition of 15 ml ice-cold buffer A. Resuspend the pellet thoroughly.
- Centrifuge the suspension at 250 x g for 10 min at RT.
- Discard the supernatant.
- To remove myelin, add 10 ml 25% (w/v) BSA (Sigma, purity > 98%) and centrifuge at 1,000 x g for 20 min at 4 °C. 25% BSA should be dissolved in 1x PBS (PAA Laboratories) and filtered through 0.2 μm filter).
- Carefully discard the supernatant, dissolve the pellet in 10 ml Buffer A and transfer to a new Falcon tube.
- Centrifuge the suspension at 250 x g for 5 min at RT. The resulting pellet contains the endothelial cells.
- Wash twice the collagen IV- coated 12-well plate with PBS.
- Resuspend the pellet in 4 ml of growth medium (DMEM containing 10% FCS, 50U/ml penicillin/streptomycin, 1% L-glutamine) and plate the cell suspension into two wells, 2 ml to each well. Add the puromycin to a final concentration of 4 μg/ml and incubate for 45 min at 37 °C in a cell culture incubator. This step allows removal of rapidly adhering cells. Note: Puromycin is added for 24 hr. Only brain ECs can metabolize it, for other cell types puromycin is toxic 14.
- Transfer the 2 ml medium containing non-adhered cells from step 1.14 into two fresh wells (these wells contain the EC fraction). Fill up the wells with adhered cells from step 1.14 with 2 ml fresh medium (these wells contain other cell types, e.g. fibroblasts, astrocytes and can be grown in parallel and used for comparisons of the morphology).
- Change the medium the following day.
2. Immortalizing the Brain Microvascular Endothelial Cells
- Cultivate the GP + E-86 Neo (GPENeo) 15 fibroblasts, secreting a replication-deficient virus with polyoma middle T oncogene in DMEM medium containing 10% heat-inactivated FCS, 50U/ml penicillin/streptomycin, and 2 mg/ml G418 (PAA Laboratories) in gelatine coated flasks. Note: Polyoma middle T oncogene transfection causes growth advantage of ECs over non-ECs leading to a homogenous monolayer of cells with endothelial morphology 4 to 6 weeks of culture. GPENeo are not commercially available and can be obtained as shared public material (please refer to original publications 4,16).
- To use the virus-containing medium for immortalization, cultivate the GPENeo cells in G418- free medium for 24 h.
- Remove 10 ml of the GPEneo supernatant and add Polybrene (hexadimethrine bromide) (Sigma) to a final concentration of 8 μg/ml, sterilize through 0.45 μm filter to remove the cellular fragments.
- Remove the growth medium from the EC cultures prepared in Part 1 and add 2 ml of GPEneo supernatant/Polybrene mixture into the wells. Repeat it the next day using a fresh GPEneo supernatant/Polybrene mixture. Note: Polybrene is used to make pores in the cell wall to facilitate the infection with the viral particles).
- Remove the GPEneo supernatant/Polybrene mixture the following day, wash the cells twice with PBS and maintain the cells in the growth medium changing it every 3 days. Upon confluence, split the cells 1:2 on collagen IV-coated plates.
- Typically, stable cerebral endothelial (cEND) cell lines should be obtained 4-5 weeks later.
3. Cultivating the Brain Microvascular Endothelial Cells
- Thaw the cells from cryoaliquot in water bath and transfer in 15 ml-Falcon with 10 ml pre-warmed medium.
- Centrifuge the suspension at 250 x g for 5 min at RT.
- Remove medium, transfer the pellet in the collagen IV coated T25 cm2 cell culture flask with pre-warmed growth medium (cell density for plating should be at least 1x 104 cells/ml).
- Change medium the next day and after the cells reached confluence (usually after 5 days) split the cells.
4. Splitting of cEND-cells (Note: Splitting Should be Done Only Once a Week, Avoid Splitting Higher than 1:4).
- Remove medium, wash the cells with PBS.
- Add 3 ml of warm trypsin-EDTA solution (PAA Laboratories) (T75 cm2 flask), incubate at 37 °C and wait until cells layer is dispersed (usually within 5 to 15 min).
- Add 5 ml of growth medium, pipette up and down, and transfer to a new collagen IV-coated flask.
5. Freezing of cEND-cells
- Obtain the cell suspension as described in step 4
- Centrifuge the suspension at 250 x g for 5 min at RT.
- Resuspend the cell pellet (obtained from 1 T75 cm2 flask) in 6 ml freezing medium (95% growth medium, 5% DMSO).
- Divide the cell suspension into four 1.5 ml cryo-aliquots. Note: One cryo-aliquot can be seeded on T25 cm2 flask.
- Store the cryo-aliquots under liquid nitrogen vapor temperature.
cEND growth medium: 450 ml DMEM, 10% FCS, 10 ml L-glutamine, 2% MEM-Kit, 2% NEAA, 10 ml natrium pyruvate, 50 U/ml penicillin/ streptomycin
6. Representative Results
The cEND and cerebEND cells were characterized by immunostainings of endothelial and BBB markers as well as by measurements of transendothelial electrical resistance (TEER) and permeability. The cEND and cerebEND had a morphology similar to primary cultures of brain ECs, with monolayers of tightly packed elongated cells that exhibited growth inhibition at confluence. The cells expressed well detectable levels of claudin-5, occludin and VE-cadherin proteins which were localized at the cell-cell junctions, as shown by immunofluorescence (Figure 3) 4,5. Culturing cEND cells in serum-reduced medium led to an increase in TEER (from 150 Ωcm2 in the presence of 10% serum to 500 Ωcm2 in the presence of 2% serum), which was potentiated by the addition of hydrocortisone (800 Ωcm2) or insulin (1,000 Ωcm2)4. Monolayers of cEND cultured in serum-reduced medium for 21 days had TEER of 900 Ωcm2 (Figure 4). TEER was measured using an assembly containing current-passing and voltage-measuring electrodes (World Precision Instruments). For comparison, the primary microvascular brain ECs has been reported to have TEER values of 200-600 Ωcm2 and a commercially available mouse cell line bEnd.3 had TEER values of 100-140 Ωcm2 (for recent review see Abbot 2005 and Toth et al., 2011 17,18). In addition, the passage of macromolecules, such as non-charged FITC-dextrans of molecular masses 4, 10, 70 and 500 kDa, or fluorescein (300 Da) across the monolayer of cEND in 2% FCS differentiation medium over 4 hr was decreased compared with control cells maintained in growth medium containing 10% FCS: paracellular flux was reduced to 30% of control cells for fluorescein, to 26% for FITC-dextrans 10 and 70 kDa, and flux was reduced to 4.5% of control cells for FITC-dextrtan 500 kDa. Similar to TEER values, the permeability was at the lowest level in the cells cultured in the presence of glucocorticoids (GC) 4. In further studies, we identified the glucocorticoid target genes, occludin, claudin-5 and VE-cadherin in brain vascular endothelium 4,7,9. GC treatment led to an increase in the expression of these proteins and to the rearrangement of VE-cadherin to the cytoskeleton. The direct GC-mediated regulation of tight junction proteins occludin and claudin-5 appears through GC response elements in their promoter regions 4,7,19. Additionally, claudin-5 was identified as a novel estrogen target in vascular endothelium 10.
Endothelial dysfunction underlies many different diseases. We cultured the cEND under oxygen/glucose deprivation (OGD) conditions. OGD led to the disruption of the BBB function, which could be reconstituted after combined treatment with GC and proteasome inhibitor bortezomib 12. OGD conditions led to a strong increase in glucose uptake and in the expression of glucose transporters in cerebEND, which could be attenuated by the addition of MK801, a non-competitive inhibitor of the NMDA-receptor 13.
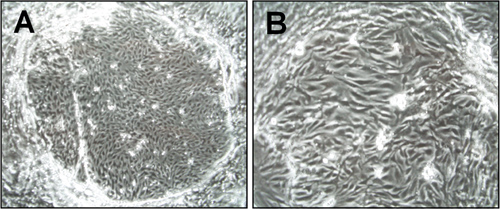
Figure 1. Morphology of brain microvascular endothelial cells (cEND) one week after isolation. Light microscopy pictures were taken under the 6x (A) and 15x (B) magnification. The island-formed colonies of endothelial cells are visible.
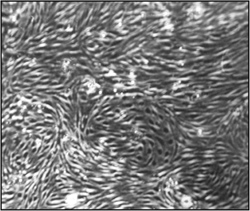
Figure 2. Morphology of brain microvascular endothelial cells (cEND) one month after isolation and immortalization. Light microscopy pictures were taken under the 15x magnification. A confluent, homogenous endothelial cell monolayer can be observed.
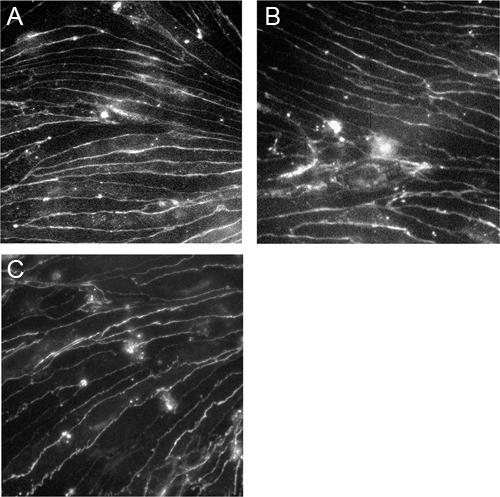
Figure 3. Immortalized brain microvascular endothelial cells express claudin-5, occludin and VE-cadherin.CEND cells were grown on collagen IV-coated coverslips and stained with antibodies against claudin-5 (A), occludin (B) and VE-cadherin (C). The images were taken using a Zeiss Axioscop2 microscope under the 40x magnification.
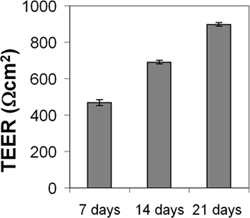
Figure 4. Measurement of transendothelial electrical resistance (TEER) of cEND monolayer. CEND were grown on collagen IV-coated transwell filters (pore size 0.4 μm). After reaching confluence, the cells were maintained in medium containing 2% FCS. TEER was measured after 7, 14 and 21 days using an assembly containing current-passing and voltage-measuring electrodes.
Discussion
The described procedure can be used for isolation of microvascular ECs from different mouse strains as well as from different knock-out mouse strains to study the specific changes in vascular endothelium function. As a method of immortalization we used the transformation with oncoprotein of murine polyomavirus, Polyoma middle T antigen. It transforms rapidly immature endothelial cells in vivo and in vitro 20,21,22. Other methods of immortalization of ECs described in the literature include for example the immortalization with SV40 large T antigen of Simian Vacuolating Virus 40 23, adenovirus gene product E1A 24 or overexpression of the catalytic subunit of human telomerase 3. The immortalization with PymT is specific for the murine immature ECs, which allows obtaining a homogenous EC culture from neonatal mice in a short time. Immortalization changes the properties of the cells and the results obtained with immortalized cell lines should be compared either with primary cells or with experiments in vivo with mice. PymT immortalized EC has been described to express high levels of fibrinolytic activity resulting from increased production of urokinase-type plasminogen activator and decreased production of plasminogen activator inhibitors 25. Direct comparison of PymT immortalized bEND5 cell line with primary ECs revealed that both in vitro BBB models are well suited for T cell adhesion studies 26.
The cell lines established according to the described procedure can be used at low passage number to maintain barrier properties by high expression of BBB and EC junctional proteins, as cells lose these properties during aging. Thus after loss of barrier properties, the preparation of a new cell line should be considered. Cell plating density should be high for ECs, as this is critical for cell proliferation. This must be considered during the isolation procedure as well as maintenance of the immortalized ECs. Each new cell line should be tested for its barrier properties and for possible contaminants with other cell types. EC monolayers can be stained with anti-galactocerebroside, anti-glial fibrillary acidic protein and anti-smooth muscle antigen as primary antibodies to exclude the contamination with oligodendrocytes, astrocytes and pericytes 4,27. To rule out contamination by meningal vasculature, the expression of thrombomodulin can be tested, which is expressed in all vascular beds with the exception of the brain 28.
Interestingly, the immortalized microvascular endothelial cell lines isolated from different brain regions differ in their barrier properties and susceptibility to pro-inflammatory stimuli. As an example, the cerebral cEND and cerebellar cerebEND cell lines can be mentioned 4,5. CerebEND showed, in comparison to cEND, lower expression levels of major tight junction components claudin-1 and occludin. However, the levels of claudin-3 and -12 were higher in cerebEND. The barrier function of cerebEND cells suffered much more under a treatment with the inflammatory mediator, TNFα than the barrier properties of cEND cells did 5. Higher cerebellar vulnerability to inflammatory stimuli, can be observed in vivo in multiple sclerosis patients and in its animal model experimental autoimmune encephalomyelitis 29. These interesting findings show the necessity of generating and characterizing individual in vitro models for different brain regions, in order to improve the future drug targeting into the brain.
Acknowledgements
This research was supported by the Deutsche Forschungsgemeinschaft DFG under grant number FO 315/4-1 and DFG SFB 688.
Materials
| Name | Company | Catalog Number | Comments |
| Name of the reagent | Company | Catalogue number | Comments |
| Bovine serum albumin (BSA) | Sigma-Aldrich | A7906 | Purity > 98% |
| collagen IV | Sigma-Aldrich | C5533 | 50 μg/ml in 50 mM acetic acid |
| Collagenase/ Dispase | Roche | 10269638001 | |
| Dulbecco's modified Eagle's medium (DMEM) | Sigma-Aldrich | D5796 | |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | D2650 | |
| Fetal Calf Serum (FCS) | PAA Laboratories | A15110-1333 | final concentration 10%, heat-inactivated (30 min at 56 °C) |
| L-glutamine | Biochrom AG | K0282 | Storage: ≤ -15 °C |
| MEM Vitamine | Biochrom AG | K0373 | Storage: ≤ -15 °C |
| Na-pyruvate | Biochrom AG | L0473 | |
| Neomycin (G418) | PAA Laboratories | P11-012 | |
| Non essential amino acids (NEA) | Biochrom AG | K0293 | Storage at 4 °C |
| Penicilin/Streptomycin | Biochrom AG | A2212 | Storage: ≤ -15 °C |
| Phosphate buffered saline (PBS) | Biochrom AG | L1825 | |
| Polybrene (hexadimethrine bromide) | Sigma-Aldrich | 107689 | 0.8 mg/ml in PBS, storage at -20 °C |
| Puromycin | Sigma-Aldrich | P8833 | |
| Trypsin/EDTA | Biochrom AG | L1825 | 0.05%, 0.02% EDTA in PBS |
References
- Wilhelm, I., Fazakas, C., Krizbai, I. A. In vitro models of the blood-brain barrier. Acta Neurobiol. Exp (Wars). 71, 113 (2011).
- Forster, C. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J. Physiol. 586, 1937 (2008).
- Weksler, B. B. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB. J. 19, 1872 (2005).
- Forster, C. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 565 (Pt 2), 475 (2005).
- Silwedel, C., Forster, C. Differential susceptibility of cerebral and cerebellar murine brain microvascular endothelial cells to loss of barrier properties in response to inflammatory stimuli. J. Neuroimmunol. 179, 37 (2006).
- Forster, C. Glucocorticoid effects on mouse microvascular endothelial barrier permeability are brain specific. J. Physiol. 573 (Pt 2), 413 (2006).
- Burek, M., Forster, C. Y. Cloning and characterization of the murine claudin-5 promoter. Mol. Cell Endocrinol. 298, 19 (2009).
- Forster, C., Kahles, T., Kietz, S., Drenckhahn, D. Dexamethasone induces the expression of metalloproteinase inhibitor TIMP-1 in the murine cerebral vascular endothelial cell line cEND. J. Physiol. 580 (Pt.3), 937 (2007).
- Blecharz, K. G., Drenckhahn, D., Forster, C. Y. Glucocorticoids increase VE-cadherin expression and cause cytoskeletal rearrangements in murine brain endothelial cEND cells. J. Cereb. Blood Flow Metab. 28, 1139 (2008).
- Burek, M., Arias-Loza, P. A., Roewer, N., Forster, C. Y. Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler. Thromb Vasc. Biol. 30, 298 (2010).
- Blecharz, K. G. Glucocorticoid effects on endothelial barrier function in the murine brain endothelial cell line cEND incubated with sera from patients with multiple sclerosis. Mult. Scler. 16, 293 (2010).
- Kleinschnitz, C. Glucocorticoid insensitivity at the hypoxic blood-brain barrier can be reversed by inhibition of the proteasome. Stroke. 42, 1081 (2011).
- Neuhaus, W. Addition of NMDA-receptor antagonist MK801 during oxygen/glucose deprivation moderately attenuates the upregulation of glucose uptake after subsequent reoxygenation in brain endothelial cells. Neurosci. Lett. 506, 44 (2012).
- Perriere, N. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J. Neurochem. 93, 279 (2005).
- Golenhofen, N., Ness, W., Wawrousek, E. F., Drenckhahn, D. Expression and induction of the stress protein alpha-B-crystallin in vascular endothelial cells. Histochem Cell Biol. 117, 203 (2002).
- Risau, W., Engelhardt, B., Wekerle, H. Immune function of the blood-brain barrier: incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J. Cell Biol. 110, 1757 (1990).
- Abbott, N. J. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol. Neurobiol. 25, 5 (2005).
- Toth, A. Patented in vitro blood-brain barrier models in CNS drug discovery. Recent Pat. CNS Drug Discov. 6, 107 (2011).
- Harke, N. Glucocorticoids regulate the human occludin gene through a single imperfect palindromic glucocorticoid response element. Mol. Cell Endocrinol. 295, 39 (2008).
- Kiefer, F., Courtneidge, S. A., Wagner, E. F. Oncogenic properties of the middle T antigens of polyomaviruses. Adv. Cancer Res. 64, 125 (1994).
- Kiefer, F. Endothelial cell transformation by polyomavirus middle T antigen in mice lacking Src-related kinases. Curr. Biol. 4, 100 (1994).
- Ong, S. H. ShcA and Grb2 mediate polyoma middle T antigen-induced endothelial transformation and Gab1 tyrosine phosphorylation. EMBO J. 20, 6327 (2001).
- O'Connell, K. A., Edidin, M. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J. Immunol. 144, 521 (1990).
- Roux, F. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J. Cell Physiol. 159, 101 (1994).
- Montesano, R. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 62, 435 (1990).
- Steiner, O., Coisne, C., Engelhardt, B., Lyck, R. Comparison of immortalized bEnd5 and primary mouse brain microvascular endothelial cells as in vitro blood-brain barrier models for the study of T cell extravasation. J. Cereb. Blood Flow Metab. 31, 315 (2010).
- Skaper, S. D., Conn, M. . Methods in Neurosciences. 2, 1303 (1990).
- Ishii, H. Thrombomodulin, an endothelial anticoagulant protein, is absent from the human brain. Blood. 67, 362 (1986).
- Minagar, A., Alexander, J. S. Blood-brain barrier disruption in multiple sclerosis. Mult. Scler. 9, 540 (2003).
Explore More Articles
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved