A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Procedure for Fabricating Biofunctional Nanofibers
In This Article
Summary
An efficient approach for preparing nanofibers decorated with functional groups capable of specifically interacting with proteins is described. The approach first requires the preparation of a polymer functionalized with the appropriate functional group. The functional polymer is fabricated into nanofibers by electrospinning. The effectiveness of the binding of the nanofibers with a protein is studied by confocal microscopy.
Abstract
Electrospinning is an effective processing method for preparing nanofibers decorated with functional groups. Nanofibers decorated with functional groups may be utilized to study material-biomarker interactions i.e. act as biosensors with potential as single molecule detectors. We have developed an effective approach for preparing functional polymers where the functionality has the capacity of specifically binding with a model protein. In our model system, the functional group is 2,4-dinitrophenyl (DNP) and the protein is anti-DNP IgE (Immunoglobulin E). The functional polymer, α,ω-bi[2,4-dinitrophenyl caproic][poly(ethylene oxide)-b-poly(2-methoxystyrene)-b-poly(ethylene oxide)] (CDNP-PEO-P2MS-PEO-CDNP), is prepared by anionic living polymerization. The difunctional initiator utilized in the polymerization was prepared by electron transfer reaction of α-methylstyrene and potassium (mirror) metal. The 2-methoxystyrene monomer was added first to the initiator, followed by the addition of the second monomer, ethylene oxide, and finally the living polymer was terminated by methanol. The α,ω-dihydroxyl polymer [HO-PEO-P2MS-PEO-OH] was reacted with N-2,4-DNP-∈-amino caproic acid, by DCC coupling, resulting in the formation of α,ω-bi[2,4-dinitrophenylcaproic][poly(ethyleneoxide)-b-poly(2-methoxystyrene)-b-poly(ethylene oxide)] (CDNP-PEO-P2MS-PEO-CDNP). The polymers were characterized by FT-IR, 1H NMR and Gel Permeation Chromatography (GPC). The molecular weight distributions of the polymers were narrow (1.1-1.2) and polymers with molecular weights greater than 50,000 was used in this study. The polymers were yellow powders and soluble in tetrahydrofuran. A water soluble CDNP-PEO-P2MS-PEO-CDNP/ DMEG (dimethoxyethylene glycol) complex binds and achieves steady state binding with solution IgE within a few seconds. Higher molecular weight (water insoluble i.e. around 50,000) CDNP-PEO-P2MS-PEO-CDNP polymers, containing 1% single wall carbon nanotubes (SWCNT) were processed into electroactive nanofibers (100 nm to 500 nm in diameter) on silicon substrate. Fluorescence spectroscopy shows that anti-DNP IgE interacts with the nanofibers by binding with the DNP functional groups decorating the fibers. These observations suggest that appropriately functionalized nanofibers hold promise for developing biomarker detection device.
Protocol
1. Synthesis of α,ω-dihydroxyl Polymer [HO-PEO-P2MS-PEO-OH]
- Assemble polymerization reactor as shown in Figure 1. The reactor for this experiment consist of a 100 ml round bottom 2-neck flask having a standard taper outer joint (Chemglass), two flow control adapters with stopcocks (Chemglass), and one Teflon stirring rod. Adapter A (Figure 1) was used to keep Ultra High Purity(UHP) Nitrogen flowing through the system in order to prevent air and moisture from entering the inert system. Adapter B (Figure 1) was used to inject the solvent, monomer, and initiator into the reaction flask.
- Dry 200 ml of Tetrahydrofuran (THF) over Na metal, using benzophenone as indicator, for a minimum of 6 hr under dry nitrogen gas.
- Dry 10 ml of 2-methoxystyrene over calcium hydride for 24 hr.
- Prepare a cold temperature bath maintained at -78 °C using a slurry of isopropanol and liquid nitrogen.
- Add 25 ml of THF into the polymerization reaction flask (see Figure 1) under nitrogen gas and keep reactor under nitrogen gas all through the polymerization.
- Place 100 ml round bottom flask into slurry.
- Add 2 ml (0.27 mmol/ ml) of the initiator solution into the reaction flask.
- Inject the first monomer, 2-methoxystyrene (4 ml) into the reaction flask.
- Allow the reaction to proceed for 40 min.
- Add 1 ml of the second monomer, ethylene oxide.
- Allow the polymerization to continue at room temperature for two days.
- Terminate the polymer with HCl (6 M)/methanol (1/20, vol/vol).
- Purify the polymer by precipitating into hexanes and dry polymer in a vacuum oven.
- Characterize the polymer using NMR.
2. Functionalization of α,ω-dihydroxyl Polymer with N-2,4-DNP-Ε-amino Caproic Acid to Obtain the Functional Polymer, CDNP-PEO-P2MS-PEO-CDNP
- In a three neck flask, place the α,ω-dihydroxyl polymer (0.05 mmol), the N-2,4-DNP-E-amino caproic acid (0.25 mmol), DCC (0.15 mmol) and DMAP (0.005 mmol) and dry on vacuum line for 4 hr.
- Distill dry dichloromethane (10 ml) into the flask.
- Release the vacuum under nitrogen and stir reaction for 12 hr at room temperature.
- Filter reaction mixture and recover polymer by precipitating twice into hexanes and methanol.
- Dry precipitated polymer in a vacuum oven at 40 °C.
- Determine the polymer structure and functionality by FT-IR and 1H NMR.
3. Preparation of CDNP-PEO-P2MS-PEO-CDNP/SWCNT Solution for Electrospinning
- Dissolve 20 w % of CDNP-PEO-P2MS-PEO-CDNP in chlorobenzene.
- Dissolve 20 w % and 40 w % of Polystyrene (MW 800,000) in chlorobenzene to prepare two solutions. The higher molecular polystyrene is used to increase polymer chain-chain entanglement and obtain the optimum viscosity required for electrospinning.
- Mix the polymer solutions prepared in 3.1 and 3.2 together to form 1:1 and 1:2 ratios of the polymers and add 1 w % Single walled carbon nanotubes (SWCNT) to the mixture and stir overnight for even distribution of CNTs.
4. Electrospinning of Polymer-CNT Composite
- Assemble the electrospinning set up as shown in Figure 2. On the right hand side of the figure is the Glassman High Voltage source. Next to it is a retort stand on which the silicon wafer is attached. To the left is another retort stand on which the syringe is mounted and behind it is the lamp for visualizing the procedure as it progresses.
- Using a hypodermic syringe, withdraw a small quantity of the CDNP-PEO-P2MS-PEO-CDNP/polystyrene/SWCNT mixture (about 1 ml) and mount the hypodermic syringe on the retort stand.
- A silicon wafer is mounted across from the syringe securely at a distance of 10 cm, and the ground clip of the high voltage source is attached to it.
- Attach the clip bearing the high voltage to be applied to the needle on the syringe, depress the plunger a little bit (to suspend a drop on the needle tip)and at this point, electrospinning is ready.
- Power on the high voltage source and adjust voltage meter to 10 kV. Depending on the nature of the polymers in the composite, higher voltages may be required, especially if nanofibers under a hundred nanometers in diameter are desired.
- Unmount silicon wafer and place in a dessicator overnight to dry completely.
5. Characterization of Nanofibers
- Initial imaging of nanofibers is done with optical microscope to observe the overall perspective of the fibers.
- Utilize Scanning Electron Microscope to observe finer details like morphology, diameter, average pore size, etc.
- Carry out further imaging with an Atomic Force Microscope to observe 3-D topography of fibers, etc.
6. Binding Specificity of Nanofibers with Anti-DNP IgE Protein
- Prepare a solution of 4 ug/l fluorescently labeled, FITC- IgE (Fluorescein Isothio- cyanate-Immunoglobulin E) in PBS-BSA (Phosphate Buffered Saline-Bovine Serum Albumin) solution.
- Place a small piece of silicon wafer on which there are nanofibers on a MatTek well coverslip. Incubate the nanofibers in this solution for one hr. Incubation is done by gently pipetting out, 10 ul IgE solution onto the silicon wafer.
- After incubation, remove unbound IgE by washing sample three times with the PBS-BSA buffer solution. The PBS solution is dispensed gently on the wall of the MatTek dish, in order to avoid squirting the buffer directly onto the nanofibers. Swirl the dish gently by hand, to distribute buffer solution on nanofibers. Carefully remove buffer with a pipette and repeat this two more times.
- For the control, incubate nanofibers in fluorescently labeled IgG (non-specific for DNP) under the same conditions.
- Visualize the bound fibers with a Confocal Microscope to observe binding with IgE. For our study, the microscope used was Leica confocal TCS SP2 with 63x lens.
7. Current-Voltage Behavior of Nanofibers
- Connect two micro positioners to a very low current source like the Keithley 6430 sensitive sourcemeter. The set up for determining the current voltage behavior is shown in Figure 3. This figure shows the Probe station used to determine the initial I-V characteristics of the nanofibers. It is made up of the Bausch and Lomb MicroZoom Microscope, a Vacuum Chuck Stage, and four Micropositioners used in probing. On the top right is the Agilent 34405A Digital Multimeter used in measuring the voltage and below that is the Keithley 6430 Sub-Femtoamp Remote Source Meter used to source the low currents that were input into the fibers.
- Mount the probe arms of the micro positioners over the fiber mat on opposite sides with the tips touching the fibers.
- Connect another two micro positioners to a Digital multimeter, mount the probe arms in-between the other two and land the tips on the fiber mat. Ensure that the four tips are as collinear as possible.
- Input varying amounts of current from the Keithley (typically in the nanoamps range).
- Measure the voltage drop across the outer tips, for every magnitude of current sourced.
- Plotting these values will indicate the type of device the fiber mat acts like.
8. Representative Results
Functional Polymer
The method for the synthesis of α,ω-bi[2,4-dinitrophenyl caproic][poly(ethylene oxide)-b-poly(2-methoxystyrene)-b-poly(ethylene oxide)] (CDNP-PEO-P2MS-PEO-CDNP) is shown in Figure 4.1 The structure of the functional polymer was confirmed by FT-IR (Figure 5) and 500 MHz 1H NMR spectroscopy (Figure 6). The FT-IR shows the complete disappearance of the -OH broad absorption around 3,500 cm-1 indicating quantitative functionalization with the CDNP group. This is also confirmed by the NMR spectrum shown in Figure 6. Using the integration of the peaks in the NMR spectrum, it was determined that the CDNP-PEO-P2MS-PEO-CDNP polymers are quantitatively functionalized.
Nanofibers
In Figure 7, a mat of conductive nanofibers obtained by electrospinning CDNP-PEO-P2MS-PEO-CDNP /polystyrene/SWCNT from chlorobenzene is shown. Confocal images obtained showed that the protein IgE binds with the DNP on the fiber surface.3 This is an indication of the specificity of binding of electrospun DNP-polymers towards IgE antibody. The intensity of light is an indicator of the presence of IgE on the nanofibers as the protein is fluorescently tagged.
Figure 8a is an AFM (Atomic Force Microscope) image of one the nanofibers obtained by this process and Figure 8b shows the dimension of this particular nanofiber is around 150 nm in diameter. By this process fibers between 100-700 nm are obtained. At this current time it is challenging to prepare fibers with a specific dimension. This is consistent with what is observed by other groups.4 Figure 9 shows SEM images of CDNP-PEO-P2MS-PEO-CDNP /polystyrene/SWCNT nanofibers and the diameter of the nanofibers were between 200 nm to 300 nm .There are three SEM images of nanofibers shown at different magnifications. Study of the three images shows the morphologies of the fibers are linear and beaded. The overall goal is to prepare fibers which are mostly linear. Figure 10 shows the I-V plot of mats of nanofibers prepared from CDNP-PEO-P2MS-PEO-CDNP /polystyrene/SWCNT. The plot shows behavior of a resistor (Ohmic). When the antigen is bound to the nanofibers, we expect to see a change In the I-V behavior of the fiber mat as this change in resistance is a characteristic which suggests that the functional fibers have potential application as the active component in sensors for single molecule detection.
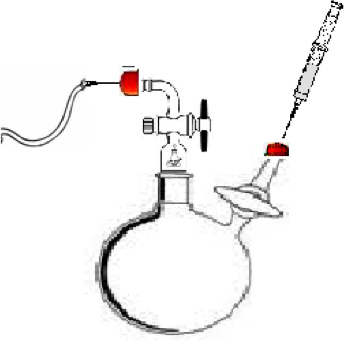
Figure 1. Polymerization reactor for synthesizing the α,ω-dihydroxyl polymer. A) The injection point for the flow UHP gas nitrogen. B.) Injection point for the solvent, monomer, and initiator. C) The reaction vessel.

Figure 2. Setup used for electrospinning using a Glassman high voltage source.
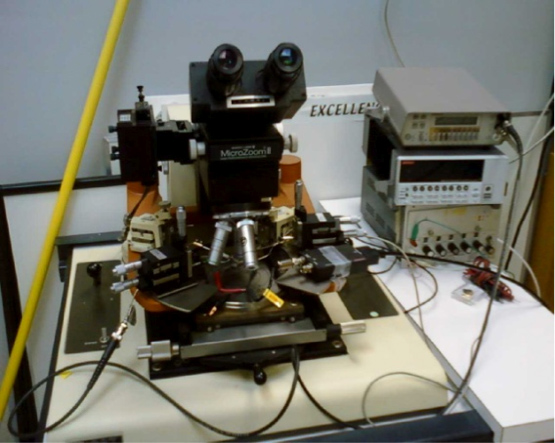
Figure 3. Setup used to measure I-V plots using a Sub-femtoamp Remote Sourcemeter (Keithley).
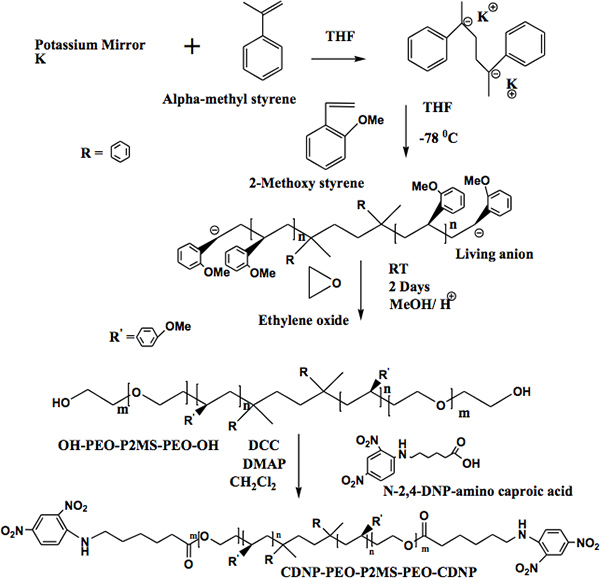
Figure 4. A).Synthetic approach for preparing OH-PEO-P2MS-PEO-OH polymers. B) Functionalization of α,ω-dihydroxy[poly(ethyleneoxide)-b-poly( 2-methoxystyrene)-b-poly(ethyleneoxide)].
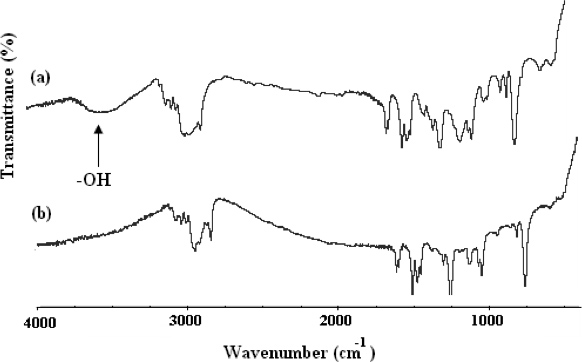
Figure 5. FT-IR spectra of (A) OH-PEO-P2MS-PEO-OH, precursor to CDNP-PEO-P2MS-PEO-CDNP and (B) CDNP-PEO-P2MS-PEO-CDNP.
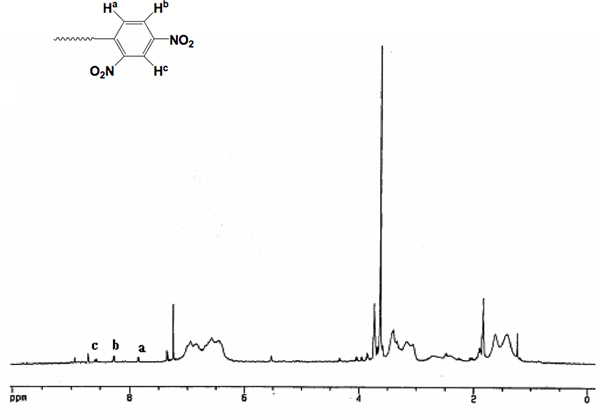
Figure 6. 500 MHz Proton NMR of CDNP-PEO-P2MS-PEO-CDNP.
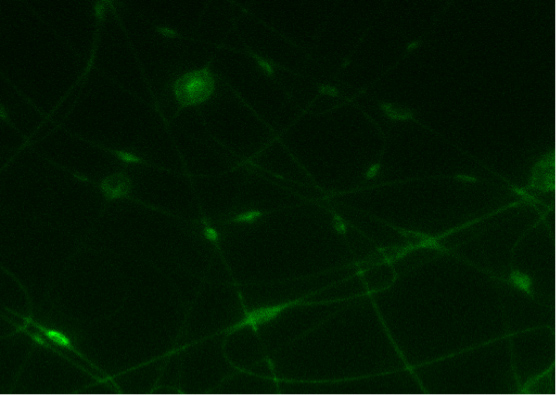
Figure 7. A) Binding image of FITC- IgE with CDNP-PEO-P2MS-PEO-CDNP fibers electrospun from chlorobenzene. B) Confocal microscope image of the control (nanofibers with IgG).
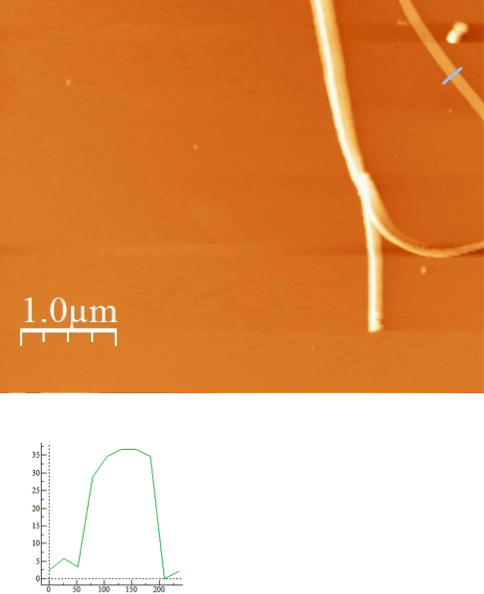
Figure 8. A) AFM image of CDNP-PEO-P2MS-PEO-CDNP Fibers electrospun from chlorobenzene and B) AFM profile i.e. dimension of one fiber shown in Figure 5a.

Figure 9. SEM images of CDNP-PEO-P2MS-PEO-CDNP /polystyrene/SWCNT nanofibers.
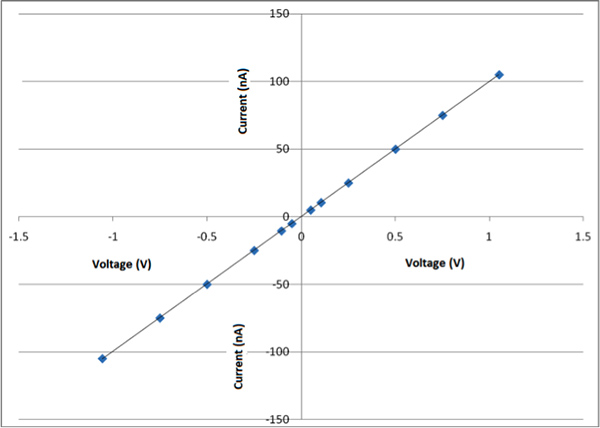
Figure 10. I-V plot of mats of nanofibers prepared from CDNP-PEO-P2MS-PEO-CDNP /polystyrene/SWCNT.
Discussion
In this report, we have presented a powerful approach for preparing biofunctional nanofibers. The nanofibers are decorated to a functional group which is specific to a model protein. The procedure and approach reported in this communication is general in nature and may be used to prepare nanofibers decorated with any functional group desired. The anionic living polymerization is powerful method to synthesize controlled polymer structures covalently connected to any number of interesting functional or functional groups wh...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was supported by NSF HRD-0630456, an NSF CREST Program and NSF is DMR-0934142.
Materials
| Name | Company | Catalog Number | Comments |
| Sodium Metal | Sigma-Aldrich | 282065 | |
| Benzophenone | Sigma-Aldrich | 239852 | |
| 2-methoxystyrene | Sigma-Aldrich | 563064 | |
| Tetrahydrofuran | Sigma-Aldrich | 178810 | |
| Chlorobenzene | Sigma-Aldrich | 319996 | |
| Single walled CNTs | Sigma-Aldrich | 704113 | |
| Polystyrene | Sigma-Aldrich | 81416 | |
| Silicon Wafers | Silicon Quest Int’l | 720200 | |
| Zeiss FESEM | Carl Zeiss Inc. | Ultra 60 | |
| Probestation with Bausch & Lomb MicroZoom II High Performance Microscope | Bausch and Lomb | ||
| Leica Scanning Confocal System | Leica Microsystems | TCS SP2 | |
| Sub-femtoamp Remote Sourcemeter | Keithley Instruments | 6430 | |
| Autoranging Digital Multimeter | Keithley Instruments | 175A | |
| Syringe Pump | Chemyx Inc. | Fusion 200 | |
| Zeiss Optical Microscope | Carl Zeiss Inc. | Zeiss/Axiotech |
References
- Sannigrahi, B., Sil, D. Synthesis and Characterization of α,ω-bi[2,4-dinitrophenyl (DNP)] poly(2-methoxystyrene) Functional Polymers. Preliminary Evaluation of the Interaction of the Functional Polymers with RBL Mast Cells. Journal of Macromolecular Science, Part A. 45, 664-671 (2008).
- Gordon, K., Sannigrahi, B. Synthesis of Optically Active Helical Poly(2-methoxystyrene). Enhancement of HeLa and Osteoblast Cell Growth on Optically Active Helical Poly(2-methoxystyrene) Surfaces. Journal of Biomaterials Science. 2, 2055-2072 (2009).
- Baird, E. J., Holowka, D. Highly Effective Poly(Ethylene Glycol) Architectures for Specific Inhibition of Immune Receptor Activation. Biochemistry. 2, 12739-12748 (2003).
- Ramakrisna, S., Fugihara, K., Lim, W. -. E., Ma, Z. . Introductions to Electrospinning and Nanofibers. , (2005).
- Kameoka, J., Craighead, H. G. Fabrication of Oriented Polymeric Nanofibers on Planar Surfaces by Electrospinning. Applied Physics Letters. 83, 371-3773 (2003).
- Ramakrishna, S., Lala, N. L. Polymer Nanofibers for Biosensor Applications. Topics in Applied Physics. 109, 377-392 (2007).
- Reuven, D., Sil, D. Archetypical Conductive Polymer Structure for Specific Interaction with Proteins. Journal of Macromolecular Science Part A: Pure and Applied Chemistry. , (2012).
- Ogunro, O., Karunwi, K. Chiral Asymmetry of Helical Polymer Nanowire. The Journal of Physical Chemistry Letters. 1, 704-707 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved