A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Thermal Measurement Techniques in Analytical Microfluidic Devices
In This Article
Summary
Here, we present three protocols for thermal measurements in microfluidic devices.
Abstract
Thermal measurement techniques have been used for many applications such as thermal characterization of materials and chemical reaction detection. Micromachining techniques allow reduction of the thermal mass of fabricated structures and introduce the possibility to perform high sensitivity thermal measurements in the micro-scale and nano-scale devices. Combining thermal measurement techniques with microfluidic devices allows performing different analytical measurements with low sample consumption and reduced measurement time by integrating the miniaturized system on a single chip. The procedures of thermal measurement techniques for particle detection, material characterization, and chemical detection are introduced in this paper.
Introduction
Three different micro-scale thermal measurement techniques are presented in this article. The three different configurations of microfluidic devices are used for thermal particle detection (TPD), thermal characterization (thermal conductivity and specific heat), and calorimetric detection of chemical reactions and interactions.
Thermal Particle Detection
Detecting and counting particles in microfluidic devices is widely used for environmental, industrial, and biological applications1. TPD is one of the novel applications of thermal measurements in microfluidic devices2. Using heat transfer for detecting and counting particles based on the particle size reduces the complexity, cost, and size of the system. In other methods, complex optics or complex electrical measurements and advanced signal processing software are used for detecting particles.
Thermal Characterization of Liquid Substances Using Micro-Calorimeter
Liquid sample thermal characterization is the second application of thermal measurement in microfluidic devices. Performing micro-scale calorimetry will reduce the sample consumption and increase the precision by offering higher repeatability compared to conventional, bulk calorimetry methods. The procedures for thermal conductivity and specific heat measurement using the on-chip micro-calorimeter device are presented elsewhere3. The details of the heat penetration time technique for thermal conductivity measurement and the thermal wave analysis (TWA) for specific heat measurements in microfluidic devices are described in the protocol section.
Calorimetric Bio-Chemical Detection in Paper-Based Microfluidic Device
Another application of thermal measurement is biochemical detection in paper-based microfluidics. The capillary action in the porous structure of paper carries the liquid and avoids bubble initiation problems in micro-channels. The most common detection mechanisms in paper-based microfluidic devices are optical or electrochemical techniques. Optical detection suffers from high complexity and the necessity of advanced image processing software to quantize the detected signal. Electrochemical detections are also limited because they can only be applied to reactions that produce active byproducts. The recently introduced calorimetric paper-based biochemical sensor platform4 takes advantage of the paper-based microfluidic system and the label-free thermal detection mechanism. The procedures of calorimetric detection of glucose using glucose oxidase (GOD) enzyme in a paper-based microfluidic platform are presented in the protocol section.
The goal of this paper is to demonstrate the capabilities of thermal measurement techniques in microfluidic devices. The device preparation, liquid sample handling and resistance temperature detector (RTD) sensor excitation and measurement are presented in the next sections.
Protocol
1. Thermal Particle Detection (TPD)
- Prepare the micro-fabricated silicon device with a thin-film silicon nitride membrane and integrated temperature sensor by micromachining, using standard semiconductor processing technology2. Rinse the fabricated device with deionized (DI) water.
Note: The fabrication method for thermal particle detector microfluidic device is explained in prior publication2. - To produce polydimethylsiloxane (PDMS) substrates with micro-channels, create an SU8 mold using standard lithography processes5.
Note: The channel size is designed for each specific particle’s dimension.- Make PDMS by mixing a 10:1 ratio of base (30 ml) and curing agent (3 ml). Pour the PDMS on to the mold and remove the bubbles by briefly exposing it to a vacuum (5-10 min).
Note: The vacuum level is not a critical value to the degasification and it should continue until gas bubbles are totally removed from mixed PDMS. - Place the mold on a hotplate (~70 °C) for 2 hr to cure the PDMS. Then peel off the PDMS very carefully so as not to damage the mold.
Note: The Vacuum level is not a critical value.
- Make PDMS by mixing a 10:1 ratio of base (30 ml) and curing agent (3 ml). Pour the PDMS on to the mold and remove the bubbles by briefly exposing it to a vacuum (5-10 min).
- Using a manual punch, punch a tight hole (1 mm) for the PTFE tube at one end. Use a large punch (2 mm) at the other end to make the PDMS a reservoir. Place the punched micro-channel on top of the device under the microscope and align the RTD at the center of the micro-channel (Figure 1A).
- In the electrical interface, connect the electrical pins at the contact pad positions and tighten up the locking screws. Make sure the height-adjustable pins (Pogo pins) sit at the correct electrode pads on the device.
- Dilute 10 µl of the concentrated PS beads in 100 µl of DI water in a 1.5 ml tube.
- To ensure the PS beads remain neutrally buoyant, add 2.7 µl of glycerol (1.26 g/cm3) to DI water to match the fluid density to the polystyrene (PS) bead density (1.05 g/cm3).
- Connect the PTFE tube to the channel at one end and the other end to a 1 ml glass syringe. Fill the glass syringe with 0.5 ml of DI water.
Note: Tight fitting made by selecting the right punch size will avoid leakage in tubes. - Place the DI water filled syringe on the computer-controlled syringe pump. Push the water (5-20 µl/min) into the channel to fill the whole channel with fluid all the way to the reservoir.
- Load 10 µl of balanced bead solution to the reservoir and introduce the bead solution to the micro-channel by changing the flow direction on syringe pump.
- Turn on the RTD by biasing 1 mA of DC current through the computer controlled source/meter while measuring the resistance by source/meter and sorting the measured data (Figure 2).
Note: During the experiment, the sensor is biased; therefore, the temperature is continuously measured until the end of the counting experiment. The RTD sensor is electrically biased by applying a DC current in the range from 100 µA to 1 mA to continuously measure the temperature until the end of the counting experiment. It is critical to select the correct current level since there is a trade-off between noise level and the detected signal amplitude. The syringe pump is used to generate the flow in micro-channel. Selecting an appropriate flow rate to perform the TPD experiment is limited to the speed of the measurement. This speed is a function of the thermal time constant of the device and electrical measurement speed. The results of thermal particle detection experiment are shown in Figure 3. - Use the developed data processing software (LabVIEW) to convert the measured resistance data to temperature using the Callendar–Van Dusen equation6.
2. Thermal Characterization of Liquid Substances Using a Micro-calorimeter
- In this process, use the on-chip calorimeter device (Figure 4A) 3 to measure the thermal diffusivity and the specific heat of the samples.
Note: On each die, there are 2 micro-calorimeter chambers (Figure 4B). Each chamber has 2 inlets and one outlet. And each chamber has a heater and a RTD sensor integrated. - Place the micro-calorimeter device on the device holder (Figure 4C). Align the device to the microfluidic inlets and outlets with the holder fittings. Place the PDMS seal layer on top of the device.
- Install electrical connection pins on the device holder and lock the holder screws.
Note: Make sure the height-adjustable Pogo pins are aligned with the electrical contact pads. - Install the microfluidic interface layer with magnetic latches to the device holder (Figure 4D). Connect the PTFE tubes to both inlets and the outlet. Connect one inlet to the sample-loaded syringe pump and close the other one, as the enthalpy is not measured in this case.
- Use a developed computer-controlled program to load the sample into the micro-channel and chambers.
Note: The program will use discontinued flow to release excessive pressure on the thin-film suspended chamber.- Load the 300 µl sample into the glass syringe and place it on the syringe pump. Use very slow (0.25 µl/min) constant flow rates for high viscosity samples (e.g., glycerol and ionic liquids). Use a glycerol sample for thermal diffusivity measurements and ionic liquids for specific heat measurements.
- Measurements
- Thermal diffusivity measurements
- Connect the measurements setup as shown in Figure 5A. Load the glycerol sample to the micro-calorimeter chamber. Run the modified computer controlled program for heat penetration time measurement.
- Use the calibrated heat penetration equation to calculate thermal diffusivity from the measured heat penetration time7:
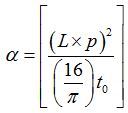
where α is thermal diffusivity, L is thickness of the chamber, p is the thickness calibration factor due to fabrication process variation, and t0 is heat penetration time.
- Specific heat measurements
- Use the TWA measurement setup as shown in Figure 5B. Use the same sample loading program and load the ionic liquid in the chamber. Run the TWA program to get the amplitude of the AC temperature fluctuations (∂TAC) and use the specific heat equation to calculate the specific, cp, heat for each ionic liquid sample8:
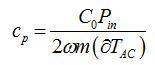
where C0 is input power calibration factor, Pin is input power, ω is frequency of the actuation signal, and m is the mass of liquid sample.
- Use the TWA measurement setup as shown in Figure 5B. Use the same sample loading program and load the ionic liquid in the chamber. Run the TWA program to get the amplitude of the AC temperature fluctuations (∂TAC) and use the specific heat equation to calculate the specific, cp, heat for each ionic liquid sample8:
- Thermal diffusivity measurements
3. Calorimetric Biochemical Detection in Paper-based Microfluidic Device
- Use microfabricated thin film (40-50 nm nickel) RTD sensor. Fabrication steps for the RTD sensor are explained in previous works4.
- For paper-based channel fabrication4, use a knife plotter to cut the paper microfluidic channels with a designed pattern (L-shape). Place the paper on top of the cutting mat, load the paper and the cutting mat to the knife plotter, and use the appropriate recipe to cut the microfluidic paper channels4.
- For device and channel integration, use an acrylic adhesive layer (5 µm) to integrate the paper on the RTD sensor. Use a clean blade to push the paper to the device and remove air bubbles (Figure 6A). The acrylic film is an adhesive layer to hold the paper over RTD sensor.
- For enzyme activation, use 50 mM sodium acetate buffer to activate the GOD enzyme. Add 1 mg of the GOD enzyme to 1 ml of sodium acetate buffer to make the 1 mg/ml solution. Adjust the pH of the solution to 5.1.
Note: Adjust the amount of acetic acid in the sodium acetate buffer to maintain the PH of solution 5.1. - Bias the RTD with 1 mA of DC current to activate the RTD and start measuring the resistance source/meter continuously while the resistance settles down after the experiment (~4 min).
Note: Figure 6B shows the measurement setup for the paper-based calorimetric test. - Introduce the 2 µl of the prepared GOD solution to the center of the paper micro-channel (immobilization site) via pipette. The detected temperature (Figure 7A) must start to decrease.
Note: This cooling effect is due to the higher operation temperature of the RTD and evaporation of the sample together. - To measure the glucose concentration, introduce standard glucose control solution9 to the channel inlet and measure the resistance change caused by the reaction. Repeat this experiment with all different glucose control solutions (high, normal and low concentrations) and save the resistance data.
- Using the temperature coefficient of resistance (TCR) for nickel RTD and Callendar–Van Dusen equation, convert the resistance change to the temperature. Calculate the concentration of the glucose in each sample by considering the reaction enthalpy of glucose and the GOD enzyme (ΔH = -80 kJ/mole) and using the concentration equation10:
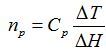
where np is detected molar concentration, Cp is heat capacity of the system and ΔT is calculated temperature.
Results
Figure 3 shows the plot of the measured thermal signal. The generated signals in the presence of the beads with corresponding optical images show the successful detection of the microsphere PS beads in the micro-channel. The thermal conductivity of the liquid passing through the micro-channel is changing due to the presence of PS beads. This change in the thermal conductivity of the channel is affecting the heat transfer in the micro-channel. The change in the heat transfer in the micro-channel is detect...
Discussion
Different thermal measurement techniques in microfluidic devices and their respective setup procedures are presented in this work. These thermal measurement methods such as thermal conductivity monitoring, thermal penetration time, amplitude of AC thermal fluctuations, and amplitude measurement of the generated heat are used to detect specific substances and investigate different reactions and interactions.
The thermal time constant plays a key role in the aforementioned thermal measurement t...
Disclosures
No conflicts of interest declared.
Acknowledgements
Partial financial support for this work was provided by the U.S. National Science Foundation through the Industry/University Cooperative Research Center on Water Equipment & Policy located at the University of Wisconsin-Milwaukee (IIP-0968887) and Marquette University (IIP-0968844). We thank Glenn M. Walker, Woo-Jin Chang and Shankar Radhakrishnan for helpful discussions.
Materials
| Name | Company | Catalog Number | Comments |
| Polydimethylsiloxane (PDMS) | Dow Corning | Sylgard 184 | |
| PS beads - 90 μm | Corpuscular | 100265 | |
| PS beads - 200 μm | Corpuscular | 100271 | |
| Glycerol | SigmaAldrich | G5516 | |
| GOD enzyme | SigmaAldrich | G7141 | |
| Glucose Control Solution - Low | Bayer contour | Low Control | |
| Glucose Control Solution - Normal | Bayer contour | Normal Control | |
| Glucose Control Solution - High | Bayer contour | High Control | |
| Chromatography filter paper | Whatman | 3001-845 | |
| Glass | VWR | 48393-106 | |
| Acrylic Film | Nitto Denko | 5600 | |
| Glass syringe (1 ml) | Hamilton | 1001 | |
| Syringe pump | New Era | NE-500 | |
| knife plotter | Silhouette | portrait | |
| Current Preamplifier | Stanford Research | SR-570 | |
| Ocilloscope | Agilent | DSO 2420A | |
| Signal Generator | HP | HP3324A | |
| Lock-in Amplifire | Stanford Research | SRS-830 | |
| Source/meter 2400 | Keithley | 2400 | |
| Source/meter 2600 | Keithley | 2436A |
References
- Zhang, H., Chon, C., Pan, X., Li, D. Methods for counting particles in microfluidic applications. Microfluid Nanofluid. 7 (6), 739-749 (2009).
- Vutha, A. K., Davaji, B., Lee, C. H., Walker, G. M. A microfluidic device for thermal particle detection. Microfluid Nanofluid. 17 (5), 871-878 (2014).
- Davaji, B., Bak, H. J., Chang, W. J., Lee, C. H. A Novel On-chip Three-dimensional Micromachined Calorimeter with Fully Enclosed and Suspended Thin-film Chamber for Thermal Characterization of Liquid Samples. Biomicrofluidics. 8 (3), 034101-034113 (2014).
- Davaji, B., Lee, C. H. A paper-based calorimetric microfluidics platform for bio-chemical sensing. Biosens. Bioelectron. 59, 120-126 (2014).
- Liu, J., et al. Process research of high aspect ratio microstructure using SU-8 resist. Microsystem Technologies. 10, 265-268 (2004).
- Dusen, M. S. V. Platinum-resistance thermometry at low temperatures. J. Am. Chem. Soc. 47 (2), 326-332 (1925).
- Arpaci, V. S. . Conduction Heat Transfer. , (1966).
- Garden, J. L., Chteau, E., Chaussy, J. Highly sensitive ac nanocalorimeter for microliter-scale liquids or biological samples. Appl. Phys. Lett. 84, 3597-3599 (2004).
- Kilo, C., et al. Evaluation of a New Blood Glucose Monitoring System with Auto-Calibration. Diabetes Technol. Ther. 7 (2), 283-294 (2005).
- Scheper, T. . Thermal Biosensors Bioactivity Bioaffinity (Advances in Biochemical Engineering/Biotechnology). , (1999).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved